Mechanism of toxicity in rotenone models of Parkinson's disease
- PMID: 14645467
- PMCID: PMC6740985
- DOI: 10.1523/JNEUROSCI.23-34-10756.2003
Mechanism of toxicity in rotenone models of Parkinson's disease
Abstract
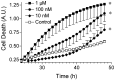
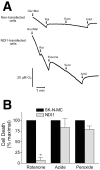
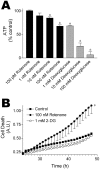
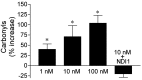
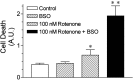
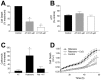
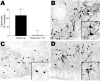
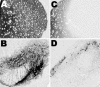
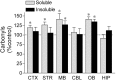
Exposure of rats to the pesticide and complex I inhibitor rotenone reproduces features of Parkinson's disease, including selective nigrostriatal dopaminergic degeneration and alpha-synuclein-positive cytoplasmic inclusions (Betarbet et al., 2000; Sherer et al., 2003). Here, we examined mechanisms of rotenone toxicity using three model systems. In SK-N-MC human neuroblastoma cells, rotenone (10 nm to 1 microm) caused dose-dependent ATP depletion, oxidative damage, and death. To determine the molecular site of action of rotenone, cells were transfected with the rotenone-insensitive single-subunit NADH dehydrogenase of Saccharomyces cerevisiae (NDI1), which incorporates into the mammalian ETC and acts as a "replacement" for endogenous complex I. In response to rotenone, NDI1-transfected cells did not show mitochondrial impairment, oxidative damage, or death, demonstrating that these effects of rotenone were caused by specific interactions at complex I. Although rotenone caused modest ATP depletion, equivalent ATP loss induced by 2-deoxyglucose was without toxicity, arguing that bioenergetic defects were not responsible for cell death. In contrast, reducing oxidative damage with antioxidants, or by NDI1 transfection, blocked cell death. To determine the relevance of rotenone-induced oxidative damage to dopaminergic neuronal death, we used a chronic midbrain slice culture model. In this system, rotenone caused oxidative damage and dopaminergic neuronal loss, effects blocked by alpha-tocopherol. Finally, brains from rotenone-treated animals demonstrated oxidative damage, most notably in midbrain and olfactory bulb, dopaminergic regions affected by Parkinson's disease. These results, using three models of increasing complexity, demonstrate the involvement of oxidative damage in rotenone toxicity and support the evaluation of antioxidant therapies for Parkinson's disease.
Figures
References
-
- Alam A, Schmidt WJ ( 2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136. - PubMed
-
- Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B ( 1997) A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem 69: 1326-1329. - PubMed
-
- Bai Y, Hajek P, Chomyn A, Chan E, Seo BB, Matsuno-Yagi A, Yagi T, Attardi G ( 2001) Lack of complex I activity in human cells carrying a mutation in MtDNA-encoded ND4 subunit is corrected by the Saccharomyces cerevisiae NADH-quinone oxidoreductase (NDI1) gene. J Biol Chem 276: 38808-38813. - PubMed
-
- Barrientos A, Moraes CT ( 1999) Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem 274: 16188-16197. - PubMed
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT ( 2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301-1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
