CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells
- PMID: 14645476
- PMCID: PMC6740966
- DOI: 10.1523/JNEUROSCI.23-34-10832.2003
CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells
Abstract
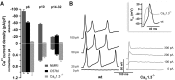
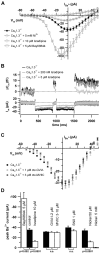
Cochlear inner hair cells (IHCs) release neurotransmitter onto afferent auditory nerve fibers in response to sound stimulation. During early development, afferent synaptic transmission is triggered by spontaneous Ca2+ spikes of IHCs, which are under efferent cholinergic control. Around the onset of hearing, large-conductance Ca2+-activated K+ channels are acquired, and Ca2+ spikes as well as the cholinergic innervation are lost. Here, we performed patch-clamp measurements in IHCs of mice lacking the CaV1.3 channel (CaV1.3-/-) to investigate the role of this prevailing voltage-gated Ca2+ channel in IHC development and synaptic function. The small Ca2+ current remaining in IHCs from 3-week-old CaV1.3-/- mice was mainly mediated by L-type Ca2+ channels, because it was sensitive to dihydropyridines but resistant to inhibitors of non-L-type Ca2+ channels such as omega-conotoxins GVIA and MVIIC and SNX-482. Depolarization induced only marginal exocytosis in CaV1.3-/- IHC, which was solely mediated by L-type Ca2+ channels, whereas robust exocytic responses were elicited by photolysis of caged Ca2+. Secretion triggered by short depolarizations was reduced proportionally to the Ca2+ current, suggesting that the coupling of the remaining channels to exocytosis was unchanged. CaV1.3-/- IHCs lacked the Ca2+ action potentials and displayed a complex developmental failure. Most strikingly, we observed a continued presence of efferent cholinergic synaptic transmission and a lack of functional large-conductance Ca2+-activated K+ channels up to 4 weeks after birth. We conclude that CaV1.3 channels are essential for normal hair cell development and synaptic transmission.
Figures



References
-
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM ( 1998) Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet 19: 264-267. - PubMed
-
- Beutner D, Voets T, Neher E, Moser T ( 2001) Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29: 681-690. - PubMed
-
- Brown AM, Kunze DL, Yatani A ( 1984) The agonist effect of dihydropyridines on Ca channels. Nature 311: 570-572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
