Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse
- PMID: 14645488
- PMCID: PMC6740976
- DOI: 10.1523/JNEUROSCI.23-34-10923.2003
Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse
Abstract
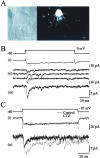
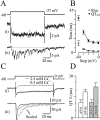
Night (scotopic) vision is mediated by a distinct retinal circuit in which the light responses of rod-driven neurons are faster than those of the rods themselves. To investigate the dynamics of synaptic transmission at the second synapse in the rod pathway, we made paired voltage-clamp recordings from rod bipolar cells (RBCs) and postsynaptic AII and A17 amacrine cells in rat retinal slices. Depolarization of RBCs from -60 mV elicited sustained Ca2+ currents and evoked AMPA receptor (AMPAR)-mediated EPSCs in synaptically coupled amacrine cells that exhibited large, rapidly rising initial peaks that decayed rapidly to smaller, steady-state levels. The transient component persisted in the absence of feedback inhibition to the RBC terminal and when postsynaptic AMPA receptor desensitization was blocked with cyclothiazide, indicating that it reflects a time-dependent decrease in the rate of exocytosis from the presynaptic terminal. The EPSC waveform was similar when RBCs were recorded in perforated-patch or whole-cell configurations, but asynchronous release from RBCs was enhanced when the intraterminal Ca2+ buffer capacity was reduced. When RBCs were depolarized from -100 mV, inactivating, low voltage-activated (T-type channel-mediated) Ca2+ currents were evident. Although Ca2+ influx through T-type channels boosted vesicle release, as reflected by larger EPSCs, it did not make the EPSCs faster, indicating that activation of T-type channels is not necessary to generate a transient phase of exocytosis. We conclude that the time course of vesicle release from RBCs is inherently transient and, together with the fast kinetics of postsynaptic AMPARs, speeds transmission at this synapse.
Figures






References
-
- Beutner D, Voets T, Neher E, Moser T ( 2001) Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29: 681-690. - PubMed
-
- Bloomfield SA, Dacheux RF ( 2001) Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20: 351-384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
