Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns
- PMID: 14645489
- PMCID: PMC6740972
- DOI: 10.1523/JNEUROSCI.23-34-10934.2003
Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns
Abstract
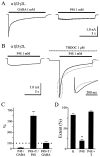
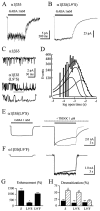
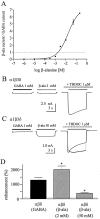
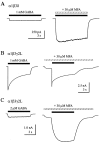
Although GABA activates synaptic (alphabetagamma) GABA(A) receptors with high efficacy, partial agonist activation of alphabetagamma isoforms and GABA activation of the primary extrasynaptic (alphabetadelta) GABA(A) receptors are limited to low-efficacy activity, characterized by minimal desensitization and brief openings. The unusual sensitivity of alphabetadelta receptor channels to neurosteroid modulation prompted investigation of whether this high sensitivity was dependent on the delta subunit or the low-efficacy channel function that it confers. We show that the isoform specificity (alphabetadelta > alphabetagamma) of neurosteroid modulation could be reversed by conditions that reversed isoform-specific activity modes, including the use of beta-alanine to achieve increased efficacy with alphabetadelta receptors and taurine to render alphabetagamma receptors low efficacy. We suggest that neurosteroids preferentially enhance low-efficacy GABA(A) receptor activity independent of subunit composition. Allosteric conversion of partial to full agonism may be a general mechanism for reversibly scaling the efficacy of GABA(A) receptors to endogenous partial agonists.
Figures


References
-
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB ( 2001) α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem 276: 38934-38939. - PubMed
-
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA ( 2001) Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol 59: 814-824. - PubMed
-
- Bianchi MT, Macdonald RL ( 2001) Mutation of the 9′ leucine in the GABAA receptor γ2L subunit produces an apparent decrease in desensitization by stabilizing open states without altering desensitized states. Neuropharmacology 41: 737-744. - PubMed
-
- Bianchi MT, Haas KF, Macdonald RL ( 2002) alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharmacology 43: 492-502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
