Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis
- PMID: 14645512
- PMCID: PMC309660
- DOI: 10.1128/MCB.23.24.8992-9002.2003
Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis
Abstract
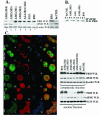
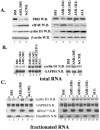
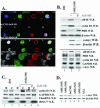
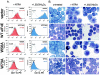
The eukaryotic translation initiation factor 4E (eIF4E) acts as both a key translation factor and as a promoter of nucleocytoplasmic transport of specific transcripts. Traditionally, its transformation capacity in vivo is attributed to its role in translation initiation in the cytoplasm. Here, we demonstrate that elevated eIF4E impedes granulocytic and monocytic differentiation. Our subsequent mutagenesis studies indicate that this block is a result of dysregulated eIF4E-dependent mRNA transport. These studies indicate that the RNA transport function of eIF4E could contribute to leukemogenesis. We extended our studies to provide the first evidence that the nuclear transport function of eIF4E contributes to human malignancy, specifically in a subset of acute and chronic myelogenous leukemia patients. We observe an increase in eIF4E-dependent cyclin D1 mRNA transport and a concomitant increase in cyclin D1 protein levels. The aberrant nuclear function of eIF4E is due to abnormally large eIF4E bodies and the loss of regulation by the proline-rich homeodomain PRH. We developed a novel tool to modulate this transport activity. The introduction of IkappaB, the repressor of NF-kappaB, leads to suppression of eIF4E, elevation of PRH, reorganization of eIF4E nuclear bodies, and subsequent downregulation of eIF4E-dependent mRNA transport. Thus, our findings indicate that this nuclear function of eIF4E can contribute to leukemogenesis by promoting growth and by impeding differentiation.
Figures
Similar articles
-
The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth.EMBO J. 2003 Feb 3;22(3):689-703. doi: 10.1093/emboj/cdg069. EMBO J. 2003. PMID: 12554669 Free PMC article.
-
Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels.Mol Cell Biol. 2005 Feb;25(3):1100-12. doi: 10.1128/MCB.25.3.1100-1112.2005. Mol Cell Biol. 2005. PMID: 15657436 Free PMC article.
-
Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities.Cancer Res. 2004 Dec 1;64(23):8639-42. doi: 10.1158/0008-5472.CAN-04-2677. Cancer Res. 2004. PMID: 15574771
-
The emerging roles of translation factor eIF4E in the nucleus.Differentiation. 2002 Mar;70(1):10-22. doi: 10.1046/j.1432-0436.2002.700102.x. Differentiation. 2002. PMID: 11963652 Review.
-
eIF4E expression in tumors: its possible role in progression of malignancies.Int J Biochem Cell Biol. 1999 Jan;31(1):59-72. doi: 10.1016/s1357-2725(98)00132-0. Int J Biochem Cell Biol. 1999. PMID: 10216944 Review.
Cited by
-
A mechanism of nucleocytoplasmic trafficking for the homeodomain protein PRH.Mol Cell Biochem. 2009 Dec;332(1-2):173-81. doi: 10.1007/s11010-009-0188-0. Epub 2009 Jul 9. Mol Cell Biochem. 2009. PMID: 19588232 Free PMC article.
-
Eukaryotic initiation factor 2alpha phosphorylation is required for B-cell maturation and function in mice.Haematologica. 2011 Sep;96(9):1261-8. doi: 10.3324/haematol.2011.042853. Epub 2011 May 12. Haematologica. 2011. PMID: 21565905 Free PMC article.
-
Identification of a novel RNA giant nuclear body in cancer cells.Oncotarget. 2016 Jan 26;7(4):4724-34. doi: 10.18632/oncotarget.6619. Oncotarget. 2016. PMID: 26678034 Free PMC article.
-
A biochemical framework for eIF4E-dependent mRNA export and nuclear recycling of the export machinery.RNA. 2017 Jun;23(6):927-937. doi: 10.1261/rna.060137.116. Epub 2017 Mar 21. RNA. 2017. PMID: 28325843 Free PMC article.
-
PRH/Hex: an oligomeric transcription factor and multifunctional regulator of cell fate.Biochem J. 2008 Jun 15;412(3):399-413. doi: 10.1042/BJ20080035. Biochem J. 2008. PMID: 18498250 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
