Calpain 3 is activated through autolysis within the active site and lyses sarcomeric and sarcolemmal components
- PMID: 14645524
- PMCID: PMC309685
- DOI: 10.1128/MCB.23.24.9127-9135.2003
Calpain 3 is activated through autolysis within the active site and lyses sarcomeric and sarcolemmal components
Abstract
Calpain 3 (Capn3) is known as the skeletal muscle-specific member of the calpains, a family of intracellular nonlysosomal cysteine proteases. This enigmatic protease has many unique features among the calpain family and, importantly, mutations in Capn3 have been shown to be responsible for limb girdle muscular dystrophy type 2A. Here we demonstrate that the Capn3 activation mechanism is similar to the universal activation of caspases and corresponds to an autolysis within the active site of the protease. We undertook a search for substrates in immature muscle cells, as several lines of evidence suggest that Capn3 is mostly in an inactive state in muscle and needs a signal to be activated. In this model, Capn3 proteolytic activity leads to disruption of the actin cytoskeleton and disorganization of focal adhesions through cleavage of several endogenous proteins. In addition, we show that titin, a previously identified Capn3 partner, and filamin C are further substrates of Capn3. Finally, we report that Capn3 colocalizes in vivo with its substrates at various sites along cytoskeletal structures. We propose that Capn3-mediated cleavage produces an adaptive response of muscle cells to external and/or internal stimuli, establishing Capn3 as a muscle cytoskeleton regulator.
Figures
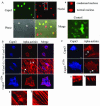
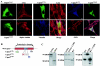
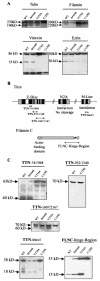
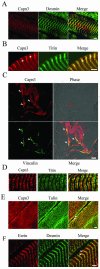

References
-
- Andreoli, C., M. Martin, R. Le Borgne, H. Reggio, and P. Mangeat. 1994. Ezrin has properties to self-associate at the plasma membrane. J. Cell Sci. 107:2509-2521. - PubMed
-
- Baghdiguian, S., M. Martin, I. Richard, F. Pons, C. Astier, N. Bourg, R. T. Hay, R. Chemaly, G. Halaby, J. Loiselet, L. V. Anderson, A. Lopez de Munain, M. Fardeau, P. Mangeat, J. S. Beckmann, and G. Lefranc. 1999. Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A. Nat. Med. 5:503-511. - PubMed
-
- Belkin, A. M., N. I. Zhidkova, F. Balzac, F. Altruda, D. Tomatis, A. Maier, G. Tarone, V. E. Koteliansky, and K. Burridge. 1996. Beta 1D integrin displaces the beta 1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J. Cell Biol. 132:211-226. - PMC - PubMed
-
- Bialkowska, K., S. Kulkarni, X. Du, D. E. Goll, T. C. Saido, and J. E. Fox. 2000. Evidence that beta3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active. J. Cell Biol. 151:685-696. - PMC - PubMed
-
- Calderwood, D. A., R. Zent, R. Grant, D. J. Rees, R. O. Hynes, and M. H. Ginsberg. 1999. The talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274:28071-28074. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
