Regulation of protein tyrosine kinase signaling by substrate degradation during brain development
- PMID: 14645539
- PMCID: PMC309695
- DOI: 10.1128/MCB.23.24.9293-9302.2003
Regulation of protein tyrosine kinase signaling by substrate degradation during brain development
Abstract
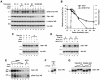
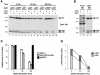
Disabled-1 (Dab1) is a cytoplasmic adaptor protein that regulates neuronal migrations during mammalian brain development. Dab1 function in vivo depends on tyrosine phosphorylation, which is stimulated by extracellular Reelin and requires Src family kinases. Reelin signaling also negatively regulates Dab1 protein levels in vivo, and reduced Dab1 levels may be part of the mechanism that regulates neuronal migration. We have made use of mouse embryo cortical neuron cultures in which Reelin induces Dab1 tyrosine phosphorylation and Src family kinase activation. We have found that Dab1 is normally stable, but in response to Reelin it becomes polyubiquitinated and degraded via the proteasome pathway. We have established that tyrosine phosphorylation of Dab1 is required for its degradation. Dab1 molecules lacking phosphotyrosine are not degraded in neurons in which the Dab1 degradation pathway is active. The requirements for Reelin-induced degradation of Dab1 in vitro correctly predict Dab1 protein levels in vivo in different mutant mice. We also provide evidence that Dab1 serine/threonine phosphorylation may be important for Dab1 tyrosine phosphorylation. Our data provide the first evidence for how Reelin down-regulates Dab1 protein expression in vivo. Dab1 degradation may be important for ensuring a transient Reelin response and may play a role in normal brain development.
Figures
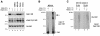


References
-
- Arnaud, L., B. A. Ballif, E. Forster, and J. A. Cooper. 2003. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr. Biol. 13:9-17. - PubMed
-
- Ballif, B. A., L. Arnaud, and J. A. Cooper. 2003. Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res. Mol. Brain Res. 117:152-159. - PubMed
-
- Beffert, U., G. Morfini, H. H. Bock, H. Reyna, S. T. Brady, and J. Herz. 2002. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J. Biol. Chem. 277:49958-49964. - PubMed
-
- Benhayon, D., S. Magdaleno, and T. Curran. 2003. Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1. Brain Res. Mol. Brain Res. 112:33-45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
