DeltaRaf-1:ER* bypasses the cyclic AMP block of extracellular signal-regulated kinase 1 and 2 activation but not CDK2 activation or cell cycle reentry
- PMID: 14645540
- PMCID: PMC309715
- DOI: 10.1128/MCB.23.24.9303-9317.2003
DeltaRaf-1:ER* bypasses the cyclic AMP block of extracellular signal-regulated kinase 1 and 2 activation but not CDK2 activation or cell cycle reentry
Abstract
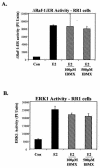
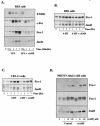
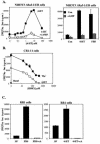
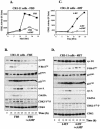
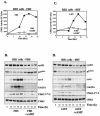
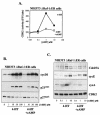
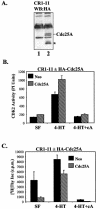
Elevation of cellular cyclic AMP (cAMP) levels inhibits cell cycle reentry in a variety of cell types. While cAMP can prevent the activation of Raf-1 and extracellular signal-regulated kinases 1 and 2 (ERK1/2) by growth factors, we now show that activation of ERK1/2 by DeltaRaf-1:ER is insensitive to cAMP. Despite this, DeltaRaf-1:ER-stimulated DNA synthesis is still inhibited by cAMP, indicating a cAMP-sensitive step downstream of ERK1/2. Although cyclin D1 expression has been proposed as an alternative target for cAMP, we found that cAMP could inhibit DeltaRaf-1:ER-induced cyclin D1 expression only in Rat-1 cells, not in CCl39 or NIH 3T3 cells. DeltaRaf-1:ER-stimulated activation of CDK2 was strongly inhibited by cAMP in all three cell lines, but cAMP had no effect on the induction of p21(CIP1). cAMP blocked the fetal bovine serum (FBS)-induced degradation of p27(KIP1); however, loss of p27(KIP1) in response to DeltaRaf-1:ER was less sensitive in CCl39 and Rat-1 cells and was completely independent of cAMP in NIH 3T3 cells. The most consistent effect of cAMP was to block both FBS- and DeltaRaf-1:ER-induced expression of Cdc25A and cyclin A, two important activators of CDK2. When CDK2 activity was bypassed by activation of the ER-E2F1 fusion protein, cAMP no longer inhibited expression of Cdc25A or cyclin A but still inhibited DNA synthesis. These studies reveal multiple points of cAMP sensitivity during cell cycle reentry. Inhibition of Raf-1 and ERK1/2 activation may operate early in G(1), but when this early block is bypassed by DeltaRaf-1:ER, cells still fail to enter S phase due to inhibition of CDK2 or targets downstream of E2F1.
Figures


Similar articles
-
Multifaceted regulation of cell cycle progression by estrogen: regulation of Cdk inhibitors and Cdc25A independent of cyclin D1-Cdk4 function.Mol Cell Biol. 2001 Feb;21(3):794-810. doi: 10.1128/MCB.21.3.794-810.2001. Mol Cell Biol. 2001. PMID: 11154267 Free PMC article.
-
Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1.Mol Cell Biol. 1997 Sep;17(9):5598-611. doi: 10.1128/MCB.17.9.5598. Mol Cell Biol. 1997. PMID: 9271435 Free PMC article.
-
cAMP inhibits linoleic acid-induced growth by antagonizing p27(kip1) depletion, but not interfering with the extracellular signal-regulated kinase or AP-1 activities.Biochim Biophys Acta. 1998 Oct 21;1405(1-3):139-46. doi: 10.1016/s0167-4889(98)00098-6. Biochim Biophys Acta. 1998. PMID: 9784625
-
An anchorage-dependent signal distinct from p42/44 MAP kinase activation is required for cell cycle progression.Oncogene. 1998 Sep 10;17(10):1271-7. doi: 10.1038/sj.onc.1202057. Oncogene. 1998. PMID: 9771970
-
Activated Raf-1 causes growth arrest in human small cell lung cancer cells.J Clin Invest. 1998 Jan 1;101(1):153-9. doi: 10.1172/JCI831. J Clin Invest. 1998. PMID: 9421477 Free PMC article.
Cited by
-
Signal control through Raf: in sickness and in health.Cell Res. 2012 Jan;22(1):14-22. doi: 10.1038/cr.2011.193. Epub 2011 Dec 6. Cell Res. 2012. PMID: 22143568 Free PMC article. Review.
-
Rafs constitute a nodal point in the regulation of embryonic endothelial progenitor cell growth and differentiation.J Cell Mol Med. 2007 Nov-Dec;11(6):1395-407. doi: 10.1111/j.1582-4934.2007.00123.x. J Cell Mol Med. 2007. PMID: 18205709 Free PMC article.
-
Sulfur dioxide inhibits vascular smooth muscle cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signaling.Cell Death Dis. 2014 May 22;5(5):e1251. doi: 10.1038/cddis.2014.229. Cell Death Dis. 2014. PMID: 24853429 Free PMC article.
-
Somatostatin and Somatostatin Receptors in Tumour Biology.Int J Mol Sci. 2023 Dec 28;25(1):436. doi: 10.3390/ijms25010436. Int J Mol Sci. 2023. PMID: 38203605 Free PMC article. Review.
-
Treating brain tumors with PDE4 inhibitors.Trends Pharmacol Sci. 2011 Jun;32(6):337-44. doi: 10.1016/j.tips.2011.02.015. Epub 2011 Mar 28. Trends Pharmacol Sci. 2011. PMID: 21450351 Free PMC article.
References
-
- Ally, S., T. Clair, D. Katsaros, G. Tortora, H. Yokozaki, R. A. Finch, T. L. Avery, and Y. S. Cho-Chung. 1989. Inhibition of growth and modulation of gene expression in human lung carcinoma in athymic mice by site-selective 8-Cl-cyclic adenosine monophosphate. Cancer Res. 49:5650-5655. - PubMed
-
- Balmanno, K., and S. J. Cook. 1999. Sustained MAP kinase activation is required for the expression of cyclin D1, p21CIP1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 18:3085-3097. - PubMed
-
- Baptist, M., F. Lamy, J. Gannon, T. Hunt, J. E. Dumont, and P. P. Roger. 1996. Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles. J. Cell Physiol. 166:256-273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
