Stability and morphology comparisons of self-assembled virus-like particles from wild-type and mutant human hepatitis B virus capsid proteins
- PMID: 14645551
- PMCID: PMC296082
- DOI: 10.1128/jvi.77.24.12950-12960.2003
Stability and morphology comparisons of self-assembled virus-like particles from wild-type and mutant human hepatitis B virus capsid proteins
Abstract
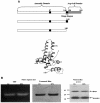
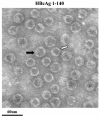
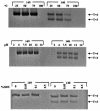
Instead of displaying the wild-type selective export of virions containing mature genomes, human hepatitis B virus (HBV) mutant I97L, changing from an isoleucine to a leucine at amino acid 97 of HBV core antigen (HBcAg), lost the high stringency of selectivity in genome maturity during virion export. To understand the structural basis of this so-called "immature secretion" phenomenon, we compared the stability and morphology of self-assembled capsid particles from the wild-type and mutant I97L HBV, in either full-length (HBcAg1-183) or truncated core protein contexts (HBcAg1-149 and HBcAg1-140). Using negative staining and electron microscopy, full-length particles appear as "thick-walled" spherical particles with little interior space, whereas truncated particles appear as "thin-walled" spherical particles with a much larger inner space. We found no significant differences in capsid stability between wild-type and mutant I97L particles under denaturing pH and temperature in either full-length or truncated core protein contexts. In general, HBV capsid particles (HBcAg1-183, HBcAg1-149, and HBcAg1-140) are very robust but will dissociate at pH 2 or 14, at temperatures higher than 75 degrees C, or in 0.1% sodium dodecyl sulfate (SDS). An unexpected upshift banding pattern of the SDS-treated full-length particles during agarose gel electrophoresis is most likely caused by disulfide bonding of the last cysteine of HBcAg. HBV capsids are known to exist in natural infection as dimorphic T=3 or T=4 icosahedral particles. No difference in the ratio between T=3 (78%) and T=4 particles (20.3%) are found between wild-type HBV and mutant I97L in the context of HBcAg1-140. In addition, we found no difference in capsid stability between T=3 and T=4 particles successfully separated by using a novel agarose gel electrophoresis procedure.
Figures
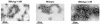








Similar articles
-
Persistence of Hepatitis B Virus DNA and the Tempos between Virion Secretion and Genome Maturation in a Mouse Model.J Virol. 2019 Oct 29;93(22):e01001-19. doi: 10.1128/JVI.01001-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462567 Free PMC article.
-
Nucleolar localization of human hepatitis B virus capsid protein.J Virol. 2004 Dec;78(24):13653-68. doi: 10.1128/JVI.78.24.13653-13668.2004. J Virol. 2004. PMID: 15564475 Free PMC article.
-
Influence of a putative intermolecular interaction between core and the pre-S1 domain of the large envelope protein on hepatitis B virus secretion.J Virol. 2002 Jul;76(13):6510-7. doi: 10.1128/jvi.76.13.6510-6517.2002. J Virol. 2002. PMID: 12050364 Free PMC article.
-
Virion Secretion of Hepatitis B Virus Naturally Occurring Core Antigen Variants.Cells. 2020 Dec 30;10(1):43. doi: 10.3390/cells10010043. Cells. 2020. PMID: 33396864 Free PMC article. Review.
-
The Structural Biology of Hepatitis B Virus: Form and Function.Annu Rev Virol. 2016 Sep 29;3(1):429-451. doi: 10.1146/annurev-virology-110615-042238. Epub 2016 Aug 1. Annu Rev Virol. 2016. PMID: 27482896 Free PMC article. Review.
Cited by
-
Secretion of genome-free hepatitis B virus--single strand blocking model for virion morphogenesis of para-retrovirus.PLoS Pathog. 2011 Sep;7(9):e1002255. doi: 10.1371/journal.ppat.1002255. Epub 2011 Sep 22. PLoS Pathog. 2011. PMID: 21966269 Free PMC article.
-
Nuclear export of human hepatitis B virus core protein and pregenomic RNA depends on the cellular NXF1-p15 machinery.PLoS One. 2014 Oct 31;9(10):e106683. doi: 10.1371/journal.pone.0106683. eCollection 2014. PLoS One. 2014. PMID: 25360769 Free PMC article.
-
PRMT5: A novel regulator of Hepatitis B virus replication and an arginine methylase of HBV core.PLoS One. 2017 Oct 24;12(10):e0186982. doi: 10.1371/journal.pone.0186982. eCollection 2017. PLoS One. 2017. PMID: 29065155 Free PMC article.
-
Hepatitis B virus capsid assembly is enhanced by naturally occurring mutation F97L.J Virol. 2004 Sep;78(17):9538-43. doi: 10.1128/JVI.78.17.9538-9543.2004. J Virol. 2004. PMID: 15308745 Free PMC article.
-
Enhanced stability of a chimeric hepatitis B core antigen virus-like-particle (HBcAg-VLP) by a C-terminal linker-hexahistidine-peptide.J Nanobiotechnology. 2018 Apr 13;16(1):39. doi: 10.1186/s12951-018-0363-0. J Nanobiotechnology. 2018. PMID: 29653575 Free PMC article.
References
-
- Beames, B., and R. E. Lanford. 1993. Carboxy-terminal truncations of the HBV core protein affect capsid formation and the apparent size of encapsidated HBV RNA. Virology 194:597-607. - PubMed
-
- Bottcher, B., S. A. Wynne, and R. A. Crowther. 1997. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature 386:88-91. - PubMed
-
- Cohen, B. J., and J. E. Richmond. 1982. Electron microscopy of hepatitis B core antigen synthesized in Escherichia coli. Nature 296:677-679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

