Further evidence that papillomavirus capsids exist in two distinct conformations
- PMID: 14645552
- PMCID: PMC296061
- DOI: 10.1128/jvi.77.24.12961-12967.2003
Further evidence that papillomavirus capsids exist in two distinct conformations
Abstract
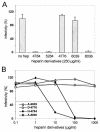
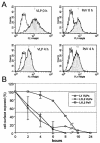
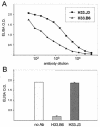
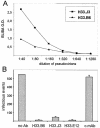
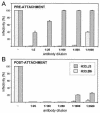
Cell surface heparan sulfate proteoglycans (HSPGs) serve as primary attachment receptors for human papillomaviruses (HPVs). To demonstrate that a biologically functional HPV-receptor interaction is restricted to a specific subset of HSPGs, we first explored the role of HSPG glucosaminoglycan side chain modifications. We demonstrate that HSPG O sulfation is essential for HPV binding and infection, whereas de-N-sulfated heparin interfered with VLP binding but not with HPV pseudoinfection. This points to differences in VLP-HSPG and pseudovirion-HSPG interactions. Interestingly, internalization kinetics of VLPs and pseudovirions, as measured by fluorescence-activated cell sorting analysis, also differ significantly with approximate half times of 3.5 and 7.5 h, respectively. These data suggest that differences in HSPG binding significantly influence postbinding events. We also present evidence that pseudovirions undergo a conformational change after cell attachment. A monoclonal antibody (H33.J3), which displays negligible effectiveness in preattachment neutralization assays, efficiently neutralizes cell-bound virions. However, no difference in H33.J3 binding to pseudovirions and VLPs was observed in enzyme-linked immunosorbent assay and virus capture assays. In contrast to antibody H33.B6, which displays equal efficiencies in pre- and postattachment neutralization assays, H33.J3 does not block VLP binding to heparin, demonstrating that it interferes with steps subsequent to virus binding. Our data strongly suggest that H33.J3 recognizes a conformation-dependent epitope in capsid protein L1, which undergoes a structural change after cell attachment.
Figures

References
-
- Bergsdorf, C., C. Beyer, V. Umansky, M. Werr, and M. Sapp. 2003. Highly efficient transport of carboxyfluorescein diacetate succinimidyl ester into COS7 cells using human papillomavirus-like particles. FEBS Lett. 536:120-124. - PubMed
-
- Bernfield, M., M. Götte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. - PubMed
-
- Booy, F. P., R. B. Roden, H. L. Greenstone, J. T. Schiller, and B. L. Trus. 1998. Two antibodies that neutralize papillomavirus by different mechanisms show distinct binding patterns at 13 Å resolution. J. Mol. Biol. 281:95-106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

