Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice
- PMID: 14645557
- PMCID: PMC296055
- DOI: 10.1128/jvi.77.24.13005-13016.2003
Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice
Abstract
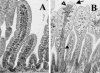
Rotavirus is the most important cause of infantile gastroenteritis. Since in vivo mucosal responses to a rotavirus infection thus far have not been extensively studied, we related viral replication in the murine small intestine to alterations in mucosal structure, epithelial cell homeostasis, cellular kinetics, and differentiation. Seven-day-old suckling BALB/c mice were inoculated with 2 x 10(4) focus-forming units of murine rotavirus and were compared to mock-infected controls. Diarrheal illness and viral shedding were recorded, and small intestinal tissue was evaluated for rotavirus (NSP4 and structural proteins)- and enterocyte-specific (lactase, SGLT1, and L-FABP) mRNA and protein expression. Morphology, apoptosis, proliferation, and migration were evaluated (immuno)histochemically. Diarrhea was observed from days 1 to 5 postinfection, and viral shedding was observed from days 1 to 10. Two peaks of rotavirus replication were observed at 1 and 4 days postinfection. Histological changes were characterized by the accumulation of vacuolated enterocytes. Strikingly, the number of vacuolated cells exceeded the number of cells in which viral replication was detectable. Apoptosis and proliferation were increased from days 1 to 7, resulting in villous atrophy. Epithelial cell turnover was significantly higher (<4 days) than that observed in controls (7 days). Since epithelial renewal occurred within 4 days, the second peak of viral replication was most likely caused by infection of newly synthesized cells. Expression of enterocyte-specific genes was downregulated in infected cells at mRNA and protein levels starting as early as 6 h after infection. In conclusion, we show for the first time that rotavirus infection induces apoptosis in vivo, an increase in epithelial cell turnover, and a shutoff of gene expression in enterocytes showing viral replication. The shutoff of enterocyte-specific gene expression, together with the loss of mature enterocytes through apoptosis and the replacement of these cells by less differentiated dividing cells, likely leads to a defective absorptive function of the intestinal epithelium, which contributes to rotavirus pathogenesis.
Figures


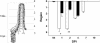




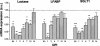
References
-
- Ball, J. M., P. Tian, C. Q. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. - PubMed
-
- Bell, L. M., H. F. Clark, E. A. O'Brien, M. J. Kornstein, S. A. Plotkin, and P. A. Offit. 1987. Gastroenteritis caused by human rotaviruses (serotype three) in a suckling mouse model. Proc. Soc. Exp. Biol. Med. 184:127-132. - PubMed
-
- Bishop, R. F., G. P. Davidson, I. H. Holmes, and B. J. Ruck. 1973. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet ii:1281-1283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

