Macrophage-tropic simian/human immunodeficiency virus chimeras use CXCR4, not CCR5, for infections of rhesus macaque peripheral blood mononuclear cells and alveolar macrophages
- PMID: 14645561
- PMCID: PMC296065
- DOI: 10.1128/jvi.77.24.13042-13052.2003
Macrophage-tropic simian/human immunodeficiency virus chimeras use CXCR4, not CCR5, for infections of rhesus macaque peripheral blood mononuclear cells and alveolar macrophages
Abstract
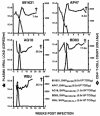
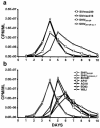
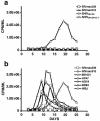
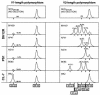
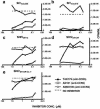
After the nearly complete and irreversible depletion of CD4(+) T lymphocytes induced by highly pathogenic simian/human immunodeficiency virus chimeric viruses (SHIVs) during infections of rhesus monkeys, tissue macrophages are able to sustain high levels (>10(6) viral RNA copies/ml) of plasma viremia for several months. We recently reported that the virus present in the plasma during the late macrophage phase of infection had acquired changes that specifically targeted the V2 region of gp120 (H. Imamichi et al., Proc. Natl. Acad. Sci. USA 99:13813-13818, 2002); some of these SHIV variants were macrophage-tropic (M-tropic). Those findings have been extended by examining the tropic properties, coreceptor usage, and gp120 structure of five independent SHIVs recovered directly from lymph nodes of late-stage animals. All of these tissue-derived SHIV isolates were able to infect alveolar macrophages. These M-tropic SHIVs used CXCR4, not CCR5, for infections of rhesus monkey PBMC and primary alveolar macrophages. Because the starting highly pathogenic T-tropic SHIV inoculum also utilized CXCR4, these results indicate that the acquisition of M-tropism in the SHIV-macaque system is not accompanied by a change in coreceptor usage. Compared to the initial T-tropic SHIV inoculum, tissue-derived M-tropic SHIVs from individual infected animals carry gp120s containing similar changes (specific amino acid deletions, substitutions, and loss of N-linked glycosylation sites), primarily within the V1 and/or V2 regions of gp120.
Figures

Similar articles
-
Amino acid deletions are introduced into the V2 region of gp120 during independent pathogenic simian immunodeficiency virus/HIV chimeric virus (SHIV) infections of rhesus monkeys generating variants that are macrophage tropic.Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13813-8. doi: 10.1073/pnas.212511599. Epub 2002 Oct 7. Proc Natl Acad Sci U S A. 2002. PMID: 12370415 Free PMC article.
-
Although macrophage-tropic simian/human immunodeficiency viruses can exhibit a range of pathogenic phenotypes, a majority of isolates induce no clinical disease in immunocompetent macaques.J Virol. 2007 Oct;81(19):10669-79. doi: 10.1128/JVI.00517-07. Epub 2007 Jul 11. J Virol. 2007. PMID: 17626082 Free PMC article.
-
Novel Strategy To Adapt Simian-Human Immunodeficiency Virus E1 Carrying env from an RV144 Volunteer to Rhesus Macaques: Coreceptor Switch and Final Recovery of a Pathogenic Virus with Exclusive R5 Tropism.J Virol. 2018 Jun 29;92(14):e02222-17. doi: 10.1128/JVI.02222-17. Print 2018 Jul 15. J Virol. 2018. PMID: 29743361 Free PMC article.
-
Understanding the basis of CD4(+) T-cell depletion in macaques infected by a simian-human immunodeficiency virus.Vaccine. 2002 May 6;20(15):1934-7. doi: 10.1016/s0264-410x(02)00072-5. Vaccine. 2002. PMID: 11983249 Review.
-
The role of viral coreceptors and enhanced macrophage tropism in human immunodeficiency virus type 1 disease progression.Sex Health. 2004;1(1):23-34. doi: 10.1071/sh03006. Sex Health. 2004. PMID: 16335478 Review.
Cited by
-
Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies.J Virol. 2012 Aug;86(16):8516-26. doi: 10.1128/JVI.00644-12. Epub 2012 May 30. J Virol. 2012. PMID: 22647691 Free PMC article.
-
In vitro Evidence That Combination Therapy With CD16-Bearing NK-92 Cells and FDA-Approved Alefacept Can Selectively Target the Latent HIV Reservoir in CD4+ CD2hi Memory T Cells.Front Immunol. 2018 Nov 5;9:2552. doi: 10.3389/fimmu.2018.02552. eCollection 2018. Front Immunol. 2018. PMID: 30455699 Free PMC article.
-
Peripheral edema with hypoalbuminemia in a nonhuman primate infected with simian-human immunodeficiency virus: a case report.J Am Assoc Lab Anim Sci. 2008 Jan;47(1):42-8. J Am Assoc Lab Anim Sci. 2008. PMID: 18210998 Free PMC article.
-
Recombination-mediated changes in coreceptor usage confer an augmented pathogenic phenotype in a nonhuman primate model of HIV-1-induced AIDS.J Virol. 2011 Oct;85(20):10617-26. doi: 10.1128/JVI.05010-11. Epub 2011 Aug 3. J Virol. 2011. PMID: 21813599 Free PMC article.
-
R5 Macrophage-Tropic HIV-1 in the Male Genital Tract.J Virol. 2015 Oct;89(20):10688-92. doi: 10.1128/JVI.01842-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223642 Free PMC article.
References
-
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
-
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. - PMC - PubMed
-
- Balotta, C., P. Bagnarelli, M. Violin, A. L. Ridolfo, D. Zhou, A. Berlusconi, S. Corvasce, M. Corbellino, M. Clementi, M. Clerici, M. Moroni, and M. Galli. 1997. Homozygous Δ32 deletion of the CCR5 chemokine receptor gene in an HIV-1-infected patient. AIDS 11:F67-F71. - PubMed
-
- Batinic, D., and F. A. Robey. 1992. The V3 region of the envelope glycoprotein of human immunodeficiency virus type 1 binds sulfated polysaccharides and CD4-derived synthetic peptides. J. Biol. Chem. 267:6664-6671. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

