Reduced cell turnover in bovine leukemia virus-infected, persistently lymphocytotic cattle
- PMID: 14645564
- PMCID: PMC296050
- DOI: 10.1128/jvi.77.24.13073-13083.2003
Reduced cell turnover in bovine leukemia virus-infected, persistently lymphocytotic cattle
Abstract
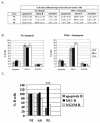
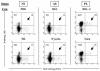
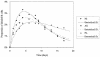
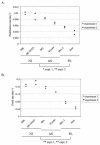
Although nucleotide analogs like bromodeoxyuridine have been extensively used to estimate cell proliferation in vivo, precise dynamic parameters are scarce essentially because of the lack of adequate mathematical models. Besides recent developments on T cell dynamics, the turnover rates of B lymphocytes are largely unknown particularly in the context of a virally induced pathological disorder. Here, we aim to resolve this issue by determining the rates of cell proliferation and death during the chronic stage of the bovine leukemia virus (BLV) infection, called bovine persistent lymphocytosis (PL). Our methodology is based on direct intravenous injection of bromodeoxyuridine in association with subsequent flow cytometry. By this in vivo approach, we show that the death rate of PL B lymphocytes is significantly reduced (average death rate, 0.057 day(-1) versus 0.156 day(-1) in the asymptomatic controls). Concomitantly, proliferation of the PL cells is also significantly restricted compared to the controls (average proliferation rate, 0.0046 day(-1) versus 0.0085 day(-1)). We conclude that bovine PL is characterized by a decreased cell turnover resulting both from a reduction of cell death and an overall impairment of proliferation. The cell dynamic parameters differ from those measured in sheep, an experimental model for BLV infection. Finally, cells expressing p24 major capsid protein ex vivo were not BrdU positive, suggesting an immune selection against proliferating virus-positive lymphocytes. Based on a comparative leukemia approach, these observations might help to understand cell dynamics during other lymphoproliferative disease such as chronic lymphocytic leukemia or human T-cell lymphotropic virus-induced adult T-cell leukemia in humans.
Figures

Similar articles
-
Bovine leukaemia virus-induced lymphocytosis in sheep is associated with reduction of spontaneous B cell apoptosis.J Gen Virol. 1997 Jan;78 ( Pt 1):153-62. doi: 10.1099/0022-1317-78-1-153. J Gen Virol. 1997. PMID: 9010299
-
Translocation of the B cell receptor to lipid rafts is inhibited in B cells from BLV-infected, persistent lymphocytosis cattle.Virology. 2003 Oct 10;315(1):135-47. doi: 10.1016/s0042-6822(03)00522-1. Virology. 2003. PMID: 14592766
-
Cattle infected with bovine leukaemia virus may not only develop persistent B-cell lymphocytosis but also persistent B-cell lymphopenia.J Vet Med B Infect Dis Vet Public Health. 2002 Aug;49(6):270-7. doi: 10.1046/j.1439-0450.2002.00559.x. J Vet Med B Infect Dis Vet Public Health. 2002. PMID: 12241026
-
Cell dynamics and immune response to BLV infection: a unifying model.Front Biosci. 2007 Jan 1;12:1520-31. doi: 10.2741/2165. Front Biosci. 2007. PMID: 17127399 Review.
-
Emphasis on cell turnover in two hosts infected by bovine leukemia virus: a rationale for host susceptibility to disease.Vet Immunol Immunopathol. 2008 Sep 15;125(1-2):1-7. doi: 10.1016/j.vetimm.2008.04.007. Epub 2008 Apr 16. Vet Immunol Immunopathol. 2008. PMID: 18513803 Review.
Cited by
-
Ablation of non-coding RNAs affects bovine leukemia virus B lymphocyte proliferation and abrogates oncogenesis.PLoS Pathog. 2020 May 14;16(5):e1008502. doi: 10.1371/journal.ppat.1008502. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32407379 Free PMC article.
-
Cytokine TNF-α and its receptors TNFRI and TNFRII play a key role in the in vitro proliferative response of BLV infected animals.Vet Res Commun. 2021 Dec;45(4):431-439. doi: 10.1007/s11259-021-09825-z. Epub 2021 Aug 27. Vet Res Commun. 2021. PMID: 34453235
-
Effects of bovine leukemia virus infection on milk neutrophil function and the milk lymphocyte profile.Vet Res. 2015 Jan 17;46(1):2. doi: 10.1186/s13567-014-0125-4. Vet Res. 2015. PMID: 25595200 Free PMC article.
-
Clone Dynamics and Its Application for the Diagnosis of Enzootic Bovine Leukosis.J Virol. 2023 Jan 31;97(1):e0154222. doi: 10.1128/jvi.01542-22. Epub 2022 Dec 19. J Virol. 2023. PMID: 36533951 Free PMC article.
-
Regulation of Expression and Latency in BLV and HTLV.Viruses. 2020 Sep 25;12(10):1079. doi: 10.3390/v12101079. Viruses. 2020. PMID: 32992917 Free PMC article. Review.
References
-
- Andritsos, L., and H. Khoury. 2002. Chronic lymphocytic leukemia. Curr. Treat. Options Oncol. 3:225-231. - PubMed
-
- Asquith, B., C. Debacq, D. Macallan, L. Willems, and C. Bangham. 2002. Lymphocyte kinetics: the interpretation of labelling data. Trends Immunol. 23:596-601. - PubMed
-
- Blaise, R., P. Masdehors, A. Lauge, D. Stoppa-Lyonnet, C. Alapetite, H. Merle-Beral, J. L. Binet, S. Omura, H. Magdelenat, L. Sabatier, and J. Delic. 2001. Chromosomal DNA and p53 stability, ubiquitin system and apoptosis in B-chronic lymphocytic leukemia lymphocytes. Leuk. Lymphoma 42:1173-1180. - PubMed
-
- Burny, A., F. Bex, H. Chantrenne, Y. Cleuter, D. Dekegel, J. Ghysdael, R. Kettmann, M. Leclercq, J. Leunen, M. Mammerickx, and D. Portetelle. 1978. Bovine leukemia virus involvement in enzootic bovine leukosis. Adv. Cancer Res. 28:251-311. - PubMed
-
- Caligaris-Cappio, F. 2000. Biology of chronic lymphocytic leukemia. Rev. Clin. Exp. Hematol. 4:5-21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

