Association of adenovirus with the microtubule organizing center
- PMID: 14645584
- PMCID: PMC296045
- DOI: 10.1128/jvi.77.24.13275-13287.2003
Association of adenovirus with the microtubule organizing center
Abstract
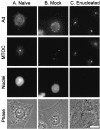
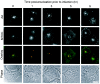
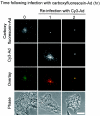
Adenoviruses (Ad) must deliver their genomes to the nucleus of the target cell to initiate an infection. Following entry into the cell and escape from the endosome, Ad traffics along the microtubule cytoskeleton toward the nucleus. In the final step in Ad trafficking, Ad must leave the microtubule and establish an association with the nuclear envelope. We hypothesized that in cells lacking a nucleus, the capsid moves to and associates with the microtubule organizing center (MTOC). To test this hypothesis, we established an experimental system to examine Ad trafficking in enucleated cells compared to Ad trafficking in intact, mock-enucleated cells. Enucleation of a monolayer of A549 human lung epithelial cells was accomplished by depolymerization of the actin cytoskeleton followed by centrifugation. Upon infection of enucleated cells with Cy3-labeled Ad, the majority of Ad capsid trafficked to a discrete, centrally located site which colocalized with pericentrin, a component of the MTOC. MTOC-associated Ad had escaped from endosomes and thus had direct access to MTOC components. Ad localization at this site was sensitive to the microtubule-depolymerizing agent nocodazole, but not to the microfilament-depolymerizing agent cytochalasin B, indicating that intact microtubules were required to maintain the localization with the MTOC. Ad localization to the MTOC in the enucleated cells was stable, as demonstrated by continuing Ad localization with pericentrin for more than 5 h after infection, a strong preference for Ad arrival at rather than Ad departure from the MTOC, and minimal redistribution of Ad between MTOCs within a single cell. In summary, the data demonstrate that the Ad capsid establishes a stable interaction with the MTOC when a nucleus is not present, suggesting that dissociation of Ad from microtubules likely requires nuclear factors.
Figures





References
-
- Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. - PubMed
-
- Bornens, M. 2002. Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14:25-34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

