Identification of proteins associated with murine gammaherpesvirus 68 virions
- PMID: 14645600
- PMCID: PMC296060
- DOI: 10.1128/jvi.77.24.13425-13432.2003
Identification of proteins associated with murine gammaherpesvirus 68 virions
Abstract
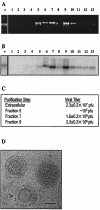
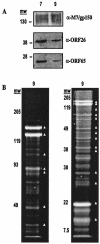
Murine gammaherpesvirus 68 (MHV68 [also known as gammaHV-68]) is distinguished by its ability to replicate to high titers in cultured cells, making it an excellent candidate for studying gammaherpesvirus virion composition. Extracellular MHV68 virions were isolated, and abundant virion-associated proteins were identified by mass spectrometry. Five nucleocapsid protein homologues, the tegument protein homologue encoded by open reading frame (ORF) 75c, and envelope glycoproteins B and H were detected. In addition, gene products from MHV68 ORF20, ORF24, ORF28, ORF45, ORF48, and ORF52 were identified in association with virions, suggesting that these gammaherpesvirus genes are involved in the early phase of infection or virion assembly and egress.
Figures

References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI046420/AI/NIAID NIH HHS/United States
- GM07185/GM/NIGMS NIH HHS/United States
- R56 AI012601/AI/NIAID NIH HHS/United States
- R01 AI029733/AI/NIAID NIH HHS/United States
- R01 CA094809/CA/NCI NIH HHS/United States
- CA83525/CA/NCI NIH HHS/United States
- CA94809/CA/NCI NIH HHS/United States
- AI29733/AI/NIAID NIH HHS/United States
- R01 CA090208/CA/NCI NIH HHS/United States
- CA90208/CA/NCI NIH HHS/United States
- T32 GM007185/GM/NIGMS NIH HHS/United States
- R01 DE014153/DE/NIDCR NIH HHS/United States
- AI12601/AI/NIAID NIH HHS/United States
- AI46420/AI/NIAID NIH HHS/United States
- CA91791/CA/NCI NIH HHS/United States
- DE14153/DE/NIDCR NIH HHS/United States
- R01 AI012601/AI/NIAID NIH HHS/United States
- R01 CA091791/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources

