Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation
- PMID: 14648237
- PMCID: PMC2538366
- DOI: 10.1007/s10162-003-4021-2
Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation
Abstract
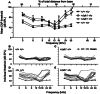
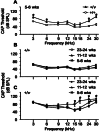
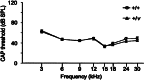
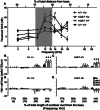
Exposure to intense noise can damage the stereocilia of sensory hair cells in the inner ear. Since stereocilia play a vital role in the transduction of sound from a mechanical stimulus into an electrical one, this pathology is thought to contribute to noise-induced hearing loss. Mice homozygous for null mutations in either the myosin VIIa ( Myo7a) or cadherin 23 ( Cdh23) genes are deaf and have disorganized stereocilia bundles. We show that mice heterozygous for a presumed null allele of Cdh23 ( Cdh23(v)) have low- and high-frequency hearing loss at 5-6 weeks of age, the high-frequency component of which worsens with increasing age. We also show that noise-induced hearing loss in 11-12-week-old Cdh23(v) heterozygotes is two times greater than for wild-type littermates. Interestingly, these effects are dependent upon the genetic background on which the Cdh23(v) mutation is carried. Noise-induced hearing loss in 11-12-week-old mice heterozygous for a null allele of Myo7a ( Myo7a(4626SB)) is not significantly different from wild-type littermates. CDH23 is the first gene known to cause deafness in the human population to be linked with predisposition to noise-induced hearing loss.
Figures


Similar articles
-
Stereocilia defects in waltzer (Cdh23), shaker1 (Myo7a) and double waltzer/shaker1 mutant mice.Hear Res. 2002 Jul;169(1-2):13-23. doi: 10.1016/s0378-5955(02)00334-9. Hear Res. 2002. PMID: 12121736
-
The effect of an age-related hearing loss gene (Ahl) on noise-induced hearing loss and cochlear damage from low-frequency noise.Hear Res. 2005 Jun;204(1-2):90-100. doi: 10.1016/j.heares.2005.01.004. Hear Res. 2005. PMID: 15925194
-
Genetic influences on susceptibility of the auditory system to aging and environmental factors.Scand Audiol Suppl. 1992;36:1-39. Scand Audiol Suppl. 1992. PMID: 1488615 Review.
-
Digenic inheritance of deafness caused by 8J allele of myosin-VIIA and mutations in other Usher I genes.Hum Mol Genet. 2012 Jun 1;21(11):2588-98. doi: 10.1093/hmg/dds084. Epub 2012 Feb 29. Hum Mol Genet. 2012. PMID: 22381527 Free PMC article.
-
[Genetic factors in susceptibility to age- and noise-related hearing loss].Pol Merkur Lekarski. 2006 Oct;21(124):384-8. Pol Merkur Lekarski. 2006. PMID: 17205784 Review. Polish.
Cited by
-
Pathological mechanisms and candidate therapeutic approaches in the hearing loss of mice carrying human MIR96 mutations.Genome Med. 2024 Oct 21;16(1):121. doi: 10.1186/s13073-024-01394-5. Genome Med. 2024. PMID: 39434156 Free PMC article.
-
Functional analysis of candidate genes from genome-wide association studies of hearing.Hear Res. 2020 Mar 1;387:107879. doi: 10.1016/j.heares.2019.107879. Epub 2020 Jan 2. Hear Res. 2020. PMID: 31927188 Free PMC article.
-
An ENU-induced mutation of Cdh23 causes congenital hearing loss, but no vestibular dysfunction, in mice.Am J Pathol. 2011 Aug;179(2):903-14. doi: 10.1016/j.ajpath.2011.04.002. Epub 2011 Jun 2. Am J Pathol. 2011. PMID: 21689626 Free PMC article.
-
Genetic variation in APE1 gene promoter is associated with noise-induced hearing loss in a Chinese population.Int Arch Occup Environ Health. 2016 May;89(4):621-8. doi: 10.1007/s00420-015-1100-8. Epub 2015 Oct 27. Int Arch Occup Environ Health. 2016. PMID: 26507517
-
Metabotropic glutamate receptors in the lateral superior olive activate TRP-like channels: age- and experience-dependent regulation.J Neurophysiol. 2007 May;97(5):3365-75. doi: 10.1152/jn.00686.2006. Epub 2007 Mar 21. J Neurophysiol. 2007. PMID: 17376850 Free PMC article.
References
-
- Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, Houdusse A, Legrain P, Wolfrum U, Richardson G, Petit C. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21(24):6689–6699. doi: 10.1093/emboj/cdf689. - DOI - PMC - PubMed
-
- Bolz H, von Brederlow B, Ramirez A, Bryda EC, Kutsche K, Nothwang HG, Seeliger M, del C-Salcedo Cabrera M, Vila MC, Molina OP, Gal A, Kubisch C. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat. Genet. 2001;27(1):108–112. doi: 10.1038/83667. - DOI - PubMed
-
- Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, Ness SL, Polomeno R, Ramesh A, Schloss M, Srisailpathy CR, Wayne S, Bellman S, Desmukh D, Ahmed Z, Khan SN, Kaloustian VM, Li XC, Lalwani A, Riazuddin S, Bitner–Glindzicz M, Nance WE, Liu XZ, Wistow G, Smith RJ, Griffith AJ, Wilcox ER, Friedman TB, Morell RJ. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. AM. J. Hum. Genet. 2001;68(l):26–37. doi: 10.1086/316954. - DOI - PMC - PubMed
-
- Bryda EC, Kim HJ, Legare ME, Frankel WN, Noben–Trauth K. High-resolution genetic and physical mapping of modifier-of-deafwaddler (mdfw) and Waltzer (Cdh23v). Genomics. 2001;73(3):338–342. - PubMed
-
- Davis AC. The prevalence of hearing impairment and reported hearing disability among adults in Great Britain. Int. J. Epidemiol. 1989;8(4):911–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

