Comparison of combination inhalers vs inhaled corticosteroids alone in moderate persistent asthma
- PMID: 14651722
- PMCID: PMC1884386
- DOI: 10.1046/j.1365-2125.2003.01887.x
Comparison of combination inhalers vs inhaled corticosteroids alone in moderate persistent asthma
Abstract
Aims: Inhalers combining long acting beta2-adrenoceptor agonists (LABA) and corticosteroids (ICS) are indicated at Step 3 of current asthma guidelines. We evaluated the relative effects of LABA + ICS combination vs ICS alone on pulmonary function, bronchoprotection, acute salbutamol recovery following methacholine bronchial challenge, and surrogate inflammatory markers in patients with moderate persistent asthma.
Methods: Twenty-nine patients with mean FEV1 (+/- SEM) of 78 +/- 3% predicted completed a randomized, double-blind, double-dummy, cross-over study. Patients received either 4 weeks of budesonide 400 microg + formoterol 12 microg (BUD + FM) combination twice daily followed by 1 week of BUD 400 microg alone twice daily, or 4 weeks of fluticasone propionate 250 microg + salmeterol 50 microg (FP + SM) combination twice daily followed by 1 week of FP 250 microg alone twice daily. Measurements were made at baseline and following each randomized treatment.
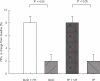
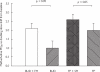
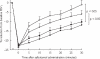
Results: FEV1 increase from pretreatment baseline as mean (+/- SEM) % predicted was significantly higher (P < 0.05) for BUD + FM (8 +/- 1%) vs BUD (2 +/- 1%), and for FP + SM (8 +/- 1%) vs FP (2 +/- 1%). The fall in FEV1 following methacholine challenge as percentage change from prechallenge baseline FEV1 was not significantly different in all four groups; BUD + FM (22 +/- 1%), BUD (24 +/- 1%), FP + SM (23 +/- 1%) and FP (23 +/- 1%). Salbutamol recovery over 30 min following methacholine challenge as area under curve (AUC %.min) was significantly blunted (P < 0.05) with BUD + FM (486.7 +/- 35.5) vs BUD (281.1 +/- 52.8), and with FP + SM (553.1 +/- 34.1) vs FP (368.3 +/- 46.7). There were no significant differences between respective combination inhalers or between respective ICS alone. Decreases in exhaled nitric oxide (NO) and serum eosinophilic cationic protein (ECP) from baseline were not significantly different between treatments.
Conclusions: Combination inhalers improve pulmonary function without potentiating anti-inflammatory effects on exhaled NO and serum ECP as compared with ICS alone, but delay acute salbutamol recovery after bronchoconstriction.
Figures


Similar articles
-
Effects of fluticasone vs. fluticasone/salmeterol on airway calibre and airway hyperresponsiveness in mild persistent asthma.Br J Clin Pharmacol. 2003 Jul;56(1):11-7. doi: 10.1046/j.1365-2125.2003.01831.x. Br J Clin Pharmacol. 2003. PMID: 12848770 Free PMC article. Clinical Trial.
-
The CONCEPT trial: a 1-year, multicenter, randomized,double-blind, double-dummy comparison of a stable dosing regimen of salmeterol/fluticasone propionate with an adjustable maintenance dosing regimen of formoterol/budesonide in adults with persistent asthma.Clin Ther. 2005 Apr;27(4):393-406. doi: 10.1016/j.clinthera.2005.03.006. Clin Ther. 2005. PMID: 15922813 Clinical Trial.
-
Real-world effects of two inhaled corticosteroid/long-acting β₂-agonist combinations in the treatment of asthma.J Asthma. 2014 Sep;51(7):762-8. doi: 10.3109/02770903.2014.905592. Epub 2014 May 13. J Asthma. 2014. PMID: 24654703 Clinical Trial.
-
Inhaled corticosteroids or long-acting beta-agonists alone or in fixed-dose combinations in asthma treatment: a systematic review of fluticasone/budesonide and formoterol/salmeterol.Clin Ther. 2009 Dec;31(12):2779-803. doi: 10.1016/j.clinthera.2009.12.021. Clin Ther. 2009. PMID: 20110019
-
Inhaled salmeterol/fluticasone propionate combination: a review of its use in persistent asthma.Drugs. 2000 Nov;60(5):1207-33. doi: 10.2165/00003495-200060050-00012. Drugs. 2000. PMID: 11129128 Review.
Cited by
-
Interactions between corticosteroids and beta2-agonists.Clin Rev Allergy Immunol. 2006 Oct-Dec;31(2-3):231-46. doi: 10.1385/CRIAI:31:2:231. Clin Rev Allergy Immunol. 2006. PMID: 17085796 Review.
-
Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events.Cochrane Database Syst Rev. 2021 Apr 14;4(4):CD007694. doi: 10.1002/14651858.CD007694.pub3. Cochrane Database Syst Rev. 2021. PMID: 33852162 Free PMC article.
-
Tiotropium in asthma: what is the evidence and how does it fit in?World Allergy Organ J. 2016 Sep 14;9(1):29. doi: 10.1186/s40413-016-0119-y. eCollection 2016. World Allergy Organ J. 2016. PMID: 27679681 Free PMC article. Review.
-
Agreement between self-reported asthma symptoms and exhaled nitric oxide levels: impact on inhaled corticosteroid prescribing in general practice. An observational study.Allergy Asthma Clin Immunol. 2019 Nov 21;15:70. doi: 10.1186/s13223-019-0390-x. eCollection 2019. Allergy Asthma Clin Immunol. 2019. PMID: 31832072 Free PMC article.
-
Tolerance to bronchodilation during treatment with long-acting beta-agonists, a randomised controlled trial.Respir Res. 2005 Sep 16;6(1):107. doi: 10.1186/1465-9921-6-107. Respir Res. 2005. PMID: 16168062 Free PMC article. Clinical Trial.
References
-
- Expert Panel Report II. Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 1997. National Asthma Education and Prevention Program.
-
- The British Guidelines on Asthma Management. 1995 review and position statement. Thorax. 1997;52(Suppl 1):S1–S21.
-
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the was adopted by the ATS Board of Directors November 1986. Am Rev Respir Dis. 1987;136:225–244. - PubMed
-
- Pauwels RA, Lofdahl CG, Postma DS, et al. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405–1411. - PubMed

