Human subcutaneous tissue distribution of fluconazole: comparison of microdialysis and suction blister techniques
- PMID: 14651730
- PMCID: PMC1884385
- DOI: 10.1046/j.1365-2125.2003.01930.x
Human subcutaneous tissue distribution of fluconazole: comparison of microdialysis and suction blister techniques
Abstract
Aims: To investigate uptake of fluconazole into the interstitial fluid of human subcutaneous tissue using the microdialysis and suction blister techniques.
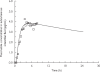
Methods: A sterile microdialysis probe (CMA/60) was inserted subcutaneously into the upper arm of five healthy volunteers following an overnight fast. Blisters were induced on the lower arm using gentle suction prior to ingestion of a single oral dose of fluconazole (200 mg). Microdialysate, blister fluid and blood were sampled over 8 h. Fluconazole concentrations were determined in each sample using a validated HPLC assay. In vivo recovery of fluconazole from the microdialysis probe was determined in each subject by perfusing the probe with fluconazole solution at the end of the 8 h sampling period. Individual in vivo recovery was used to calculate fluconazole concentrations in subcutaneous interstitial fluid. A physiologically based pharmacokinetic (PBPK) model was used to predict fluconazole concentrations in human subcutaneous interstitial fluid.
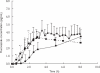
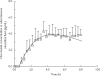
Results: There was a lag-time (approximately 0.5 h) between detection of fluconazole in microdialysate compared with plasma in each subject. The in vivo recovery of fluconazole from the microdialysis probe ranged from 57.0 to 67.2%. The subcutaneous interstitial fluid concentrations obtained by microdialysis were very similar to the unbound concentrations of fluconazole in plasma with maximum concentration of 4.29 +/- 1.19 microg ml(-1) in subcutaneous interstitial fluid and 3.58 +/- 0.14 microg ml(-1) in plasma. Subcutaneous interstitial fluid-to-plasma partition coefficient (Kp) of fluconazole was 1.16 +/- 0.22 (95% CI 0.96, 1.35). By contrast, fluconazole concentrations in blister fluid were significantly lower (P < 0.05, paired t-test) than unbound plasma concentrations over the first 3 h and maximum concentrations in blister fluid had not been achieved at the end of the sampling period. There was good agreement between fluconazole concentrations derived from microdialysis sampling and those estimated using a blood flow-limited PBPK model.
Conclusions: Microdialysis and suction blister techniques did not yield comparable results. It appears that microdialysis is a more appropriate technique for studying the rate of uptake of fluconazole into subcutaneous tissue. PBPK model simulation suggested that the distribution of fluconazole into subcutaneous interstitial fluid is dependent on tissue blood flow.
Figures

References
-
- St Georgiev V. Membrane transporters and antifungal drug resistance. Curr Drug Targets. 2000;1:261–284. - PubMed
-
- Grant SM, Clissold SP. Fluconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs. 1990;39:877–916. - PubMed
-
- Upton RN, Runciman WB, Mather LE. Regional pharmacokinetics. II. Experimental methods. Biopharm Drug Dispos. 1990;11:741–752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

