Patterns and functions of STAT activation during Drosophila embryogenesis
- PMID: 14654218
- PMCID: PMC3090291
- DOI: 10.1016/j.mod.2003.09.004
Patterns and functions of STAT activation during Drosophila embryogenesis
Abstract
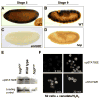
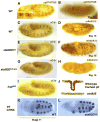
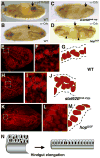
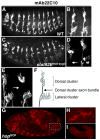
The JAK/STAT pathway mediates cytokine signaling in mammals and is involved in the function and development of the hematopoietic and immune systems. To investigate the biological functions of the JAK/STAT pathway during Drosophila development, we examined the tissue-specific localization of the tyrosine-phosphorylated, or activated form of Drosophila STAT, STAT92E. Here we show that during Drosophila embryonic development STAT92E activation is prominently detected in multiple tissues and in different developmental stages. These tissues include the tracheal pits, elongating intestinal tracks, and growing axons. We demonstrate that stat92E mutants are defective in tracheal formation, hindgut elongation, and nervous system development. Conversely, STAT92E overactivation caused premature development of the tracheal and nervous systems, and over-elongation of the hindgut. These results suggest that STAT activation is involved in proper differentiation and morphogenesis of multiple tissues during Drosophila embryogenesis.
Figures

Similar articles
-
Localized JAK/STAT signaling is required for oriented cell rearrangement in a tubular epithelium.Development. 2003 Jan;130(1):135-45. doi: 10.1242/dev.00202. Development. 2003. PMID: 12441298
-
Cyclin D-Cdk4 and cyclin E-Cdk2 regulate the Jak/STAT signal transduction pathway in Drosophila.Dev Cell. 2003 Feb;4(2):179-90. doi: 10.1016/s1534-5807(03)00024-8. Dev Cell. 2003. PMID: 12586062
-
mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family.Genes Dev. 2002 Feb 1;16(3):388-98. doi: 10.1101/gad.955202. Genes Dev. 2002. PMID: 11825879 Free PMC article.
-
The roles of the Drosophila JAK/STAT pathway.Oncogene. 2000 May 15;19(21):2598-606. doi: 10.1038/sj.onc.1203482. Oncogene. 2000. PMID: 10851058 Review.
-
The JAK-STAT pathway in Drosophila.Trends Genet. 1997 Mar;13(3):105-10. doi: 10.1016/s0168-9525(97)01006-8. Trends Genet. 1997. PMID: 9066269 Review.
Cited by
-
An efficient approach to isolate STAT regulated enhancers uncovers STAT92E fundamental role in Drosophila tracheal development.Dev Biol. 2010 Apr 15;340(2):571-82. doi: 10.1016/j.ydbio.2010.02.015. Epub 2010 Feb 18. Dev Biol. 2010. PMID: 20171201 Free PMC article.
-
Stat3 Has a Different Role in Axon Growth During Development Than It Does in Axon Regeneration After Injury.Mol Neurobiol. 2024 Mar;61(3):1753-1768. doi: 10.1007/s12035-023-03644-w. Epub 2023 Sep 29. Mol Neurobiol. 2024. PMID: 37775721
-
A gain-of-function screen for genes that influence axon guidance identifies the NF-kappaB protein dorsal and reveals a requirement for the kinase Pelle in Drosophila photoreceptor axon targeting.Genetics. 2007 Aug;176(4):2247-63. doi: 10.1534/genetics.107.072819. Epub 2007 Jul 1. Genetics. 2007. PMID: 17603113 Free PMC article.
-
Drosophila Jak/STAT Signaling: Regulation and Relevance in Human Cancer and Metastasis.Int J Mol Sci. 2018 Dec 14;19(12):4056. doi: 10.3390/ijms19124056. Int J Mol Sci. 2018. PMID: 30558204 Free PMC article. Review.
-
MANF silencing, immunity induction or autophagy trigger an unusual cell type in metamorphosing Drosophila brain.Cell Mol Life Sci. 2015 May;72(10):1989-2004. doi: 10.1007/s00018-014-1789-7. Epub 2014 Dec 16. Cell Mol Life Sci. 2015. PMID: 25511196 Free PMC article.
References
-
- Aaronson DS, Horvath CM. A road map for those who know JAK–STAT. Science. 2002;296:1653–1655. - PubMed
-
- Baksa K, Parke T, Dobens LL, Dearolf CR. The Drosophila STAT protein, stat92E, regulates follicle cell differentiation during oogenesis. Dev Biol. 2002;243:166–175. - PubMed
-
- Beccari S, Teixeira L, Rorth P. The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mech Dev. 2002;111:115–123. - PubMed
-
- Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev. 1994;8:300–312. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

