A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease
- PMID: 14655926
- PMCID: PMC4418658
- DOI: 10.1093/sleep/26.7.893
A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease
Abstract
Objectives: To determine the safety and efficacy of 2 dose formulations of melatonin for the treatment of insomnia in patients with Alzheimer's disease.
Design: A multicenter, randomized, placebo-controlled clinical trial of 2 dose formulations of oral melatonin coordinated by the National Institute of Aging-funded Alzheimer's Disease Cooperative Study. Subjects with Alzheimer's disease and nighttime sleep disturbance were randomly assigned to 1 of 3 treatment groups: placebo, 2.5-mg slow-release melatonin, or 10-mg melatonin.
Setting: Private homes and long-term care facilities.
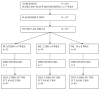
Participants: 157 individuals were recruited by 36 Alzheimer's disease research centers. Subjects with a diagnosis of Alzheimer's disease were eligible if they averaged less than 7 hours of sleep per night (as documented by wrist actigraphy) and had 2 or more episodes per week of nighttime awakenings reported by the caregiver.
Measurements: Nocturnal total sleep time, sleep efficiency, wake-time after sleep onset, and day-night sleep ratio during 2- to 3-week baseline and 2-month treatment periods. Sleep was defined by an automated algorithmic analysis of wrist actigraph data.
Results: No statistically significant differences in objective sleep measures were seen between baseline and treatment periods for the any of the 3 groups. Nonsignificant trends for increased nocturnal total sleep time and decreased wake after sleep onset were observed in the melatonin groups relative to placebo. Trends for a greater percentage of subjects having more than a 30-minute increase in nocturnal total sleep time in the 10-mg melatonin group and for a decline in the day-night sleep ratio in the 2.5-mg sustained-release melatonin group, compared to placebo, were also seen. On subjective measures, caregiver ratings of sleep quality showed improvement in the 2.5-mg sustained-release melatonin group relative to placebo. There were no significant differences in the number or seriousness of adverse events between the placebo and melatonin groups.
Conclusions: Based on actigraphy as an objective measure of sleep time, melatonin is not an effective soporific agent in people with Alzheimer's disease.
Conflict of interest statement
No significant financial interest/other relationship to disclose.
Figures


References
-
- Swearer J, Drachman D, O’Donnell B, Mitchell A. Troublesome and disruptive behaviors in dementia. J Am Geriatr Soc. 1988;36:784–90. - PubMed
-
- Pollack CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatri Psychiatry Neurol. 1991;4:204–10. - PubMed
-
- Prinz PN, Vitaliano PP, Vitiello MV, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3:299–309. - PubMed
-
- Vitiello MV, Prinz PN. Alzheimer’s disease: sleep and sleep/wake patterns. Clin Geriatr Med. 1989;5:289–99. - PubMed
-
- Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol: Med Sci. 1990;45:M131–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

