Arkadia amplifies TGF-beta superfamily signalling through degradation of Smad7
- PMID: 14657019
- PMCID: PMC291827
- DOI: 10.1093/emboj/cdg632
Arkadia amplifies TGF-beta superfamily signalling through degradation of Smad7
Abstract
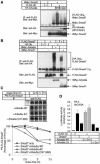
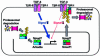
Arkadia was originally identified as a protein that enhances signalling activity of Nodal and induces mammalian nodes during early embryogenesis; however, the mechanisms by which Arkadia affects transforming growth factor-beta (TGF-beta) superfamily signalling have not been determined. Here we show that Arkadia is widely expressed in mammalian tissues, and that it enhances both TGF-beta and bone morphogenetic protein (BMP) signalling. Arkadia physically interacts with inhibitory Smad, Smad7, and induces its poly-ubiquitination and degradation. In contrast to Smurf1, which interacts with TGF-beta receptor complexes through Smad7 and degrades them, Arkadia fails to associate with TGF-beta receptors. In contrast to Smad7, expression of Arkadia is down-regulated by TGF-beta. Silencing of the Arkadia gene resulted in repression of transcriptional activities induced by TGF-beta and BMP, and accumulation of the Smad7 protein. Arkadia may thus play an important role as an amplifier of TGF-beta superfamily signalling under both physiological and pathological conditions.
Figures







References
-
- Bonni S., Wang,H.R., Causing,C.G., Kavsak,P., Stroschein,S.L., Luo,K. and Wrana,J.L. (2001) TGF-β induces assembly of a Smad2–Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat. Cell Biol., 3, 587–595. - PubMed
-
- Derynck R. and Zhang,Y.E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature, 425, 577–584. - PubMed
-
- Ebisawa T., Fukuchi,M., Murakami,G., Chiba,T., Tanaka,K., Imamura,T. and Miyazono,K. (2001) Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem., 276, 12477–12480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

