A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy
- PMID: 14657020
- PMCID: PMC291832
- DOI: 10.1093/emboj/cdg637
A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy
Abstract
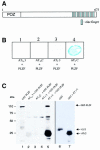
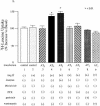
We describe a novel signaling mechanism mediated by the G-protein-coupled receptor (GPCR) angiotensin II (Ang II) type 2 receptor (AT(2)). Yeast two-hybrid studies and affinity column binding assay show that the isolated AT(2) C-terminus binds to the transcription factor promyelocytic zinc finger protein (PLZF). Cellular studies employing confocal microscopy show that Ang II stimulation induces cytosolic PLZF to co-localize with AT(2) at the plasma membrane, then drives AT(2) and PLZF to internalize. PLZF slowly emerges in the nucleus whereas AT(2) accumulates in the perinuclear region. Nuclear PLZF binds to a consensus sequence of the phosphatidylinositol-3 kinase p85 alpha subunit (p85 alpha PI3K) gene. AT(2) enhances expression of p85 alpha PI3K followed by enhanced p70(S6) kinase, essential to protein synthesis. An inactive mutant of PLZF abolishes this effect. PLZF is expressed robustly in the heart in contrast to many other tissues. This cardiac selective pathway involving AT(2), PLZF and p85 alpha PI3K may explain the absence of a cardiac hypertrophic response in AT(2) gene-deleted mice.
Figures






Similar articles
-
Promyelocytic leukemia zinc finger protein activates GATA4 transcription and mediates cardiac hypertrophic signaling from angiotensin II receptor 2.PLoS One. 2012;7(4):e35632. doi: 10.1371/journal.pone.0035632. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558183 Free PMC article.
-
A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein.Circ Res. 2006 Dec 8;99(12):1355-66. doi: 10.1161/01.RES.0000251700.00994.0d. Epub 2006 Nov 2. Circ Res. 2006. PMID: 17082479
-
Promyelocytic leukaemia zinc finger protein (PLZF) is a glucocorticoid- and progesterone-induced transcription factor in human endometrial stromal cells and myometrial smooth muscle cells.Mol Hum Reprod. 2003 Oct;9(10):611-23. doi: 10.1093/molehr/gag080. Mol Hum Reprod. 2003. PMID: 12970399
-
Molecular mechanisms of angiotensin II in modulating cardiac function: intracardiac effects and signal transduction pathways.J Mol Cell Cardiol. 1997 Nov;29(11):2893-902. doi: 10.1006/jmcc.1997.0524. J Mol Cell Cardiol. 1997. PMID: 9405164 Review.
-
Regulation of the FoxO family of transcription factors by phosphatidylinositol-3 kinase-activated signaling.Arch Biochem Biophys. 2002 Jul 15;403(2):292-8. doi: 10.1016/s0003-9861(02)00207-2. Arch Biochem Biophys. 2002. PMID: 12139979 Review. No abstract available.
Cited by
-
How does angiotensin AT(2) receptor activation help neuronal differentiation and improve neuronal pathological situations?Front Endocrinol (Lausanne). 2012 Dec 19;3:164. doi: 10.3389/fendo.2012.00164. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 23267346 Free PMC article.
-
Novel Pharmacology Following Heteromerization of the Angiotensin II Type 2 Receptor and the Bradykinin Type 2 Receptor.Front Endocrinol (Lausanne). 2022 May 26;13:848816. doi: 10.3389/fendo.2022.848816. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721749 Free PMC article.
-
Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production.Am J Physiol Renal Physiol. 2009 Jun;296(6):F1484-93. doi: 10.1152/ajprenal.90766.2008. Epub 2009 Feb 25. Am J Physiol Renal Physiol. 2009. PMID: 19244399 Free PMC article.
-
Genetically modified mouse models used for studying the role of the AT2 receptor in cardiac hypertrophy and heart failure.J Biomed Biotechnol. 2011;2011:141039. doi: 10.1155/2011/141039. Epub 2011 Apr 27. J Biomed Biotechnol. 2011. PMID: 21541238 Free PMC article. Review.
-
The intracrine renin-angiotensin system.Clin Sci (Lond). 2012 Sep;123(5):273-84. doi: 10.1042/CS20120089. Clin Sci (Lond). 2012. PMID: 22590974 Free PMC article. Review.
References
-
- Albagli O. et al. (1999) Overexpressed BCL6 (LAZ3) oncoprotein triggers apoptosis, delays S phase progression and associates with replication foci. Oncogene. 18, 5063–5075. - PubMed
-
- Barna M., Hawe,N., Niswander,L. and Pandolfi,P.P. (2000) Plzf regulates limb and axial skeletal patterning. Nat. Genet., 25, 166–172. - PubMed
-
- Chen Z., Brand,N.J., Chen,A., Chen,S.J., Tong,J.H., Wang,Z.Y., Waxman,S. and Zelent,A. (1993) Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-α locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J., 12, 1161–1167. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

