Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis
- PMID: 14657024
- PMCID: PMC291817
- DOI: 10.1093/emboj/cdg622
Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis
Abstract
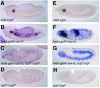
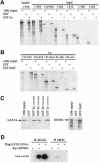
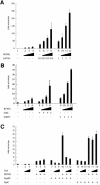
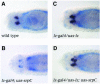
Members of the GATA and RUNX families of genes appear to have conserved functions during hematopoiesis from Drosophila to mammals. In Drosophila, the GATA factor Serpent (Srp) is required in blood cell progenitors for the formation of the two populations of blood cells (plasmatocytes and crystal cells), while the RUNX factor Lozenge (Lz) is specifically required for crystal cell development. Here we investigate the function and the mechanisms of action of Lz during hematopoiesis. Our results indicate that Lz can trigger crystal cell development. Interestingly, we show that Lz function is strictly dependent on the presence of functional Srp and that Srp and Lz cooperate to induce crystal cell differentiation in vivo. Furthermore, we show that Srp and Lz directly interact in vitro and that this interaction is conserved between Drosophila and mammals. Moreover, both Srp and mouse GATA1 synergize with mouse RUNX1 to activate transcription. We propose that interaction and cooperation between GATA and RUNX factors may play an important role in regulating blood cell formation from Drosophila to mammals.
Figures



References
-
- Alfonso T.B. and Jones,B.W. (2002) gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev. Biol., 248, 369–383. - PubMed
-
- Bernardoni R., Vivancos,B. and Giangrande,A. (1997) glide/gcm is expressed and required in the scavenger cell lineage. Dev. Biol., 191, 118–130. - PubMed
-
- Bourbon H.M. et al. (2002) A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech. Dev., 110, 71–83. - PubMed
-
- Elagib K.E., Racke,F.K., Mogass,M., Khetawat,R., Delehanty,L.L. and Goldfarb,A.N. (2003) RUNX1 and GATA-1 coexpression and cooperation in megakaryocytic differentiation. Blood, 101, 4333–4341. - PubMed
-
- Evans C.J. and Banerjee,U. (2003) Transcriptional regulation of hematopoiesis in Drosophila. Blood Cells Mol. Dis., 30, 223–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

