Activity-dependent feedforward inhibition modulates synaptic transmission in a spinal locomotor network
- PMID: 14657166
- PMCID: PMC6741052
- DOI: 10.1523/JNEUROSCI.23-35-11085.2003
Activity-dependent feedforward inhibition modulates synaptic transmission in a spinal locomotor network
Abstract
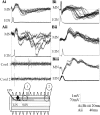
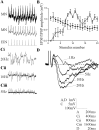
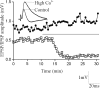
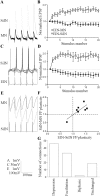
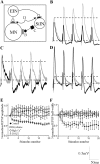
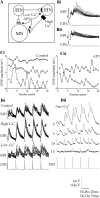
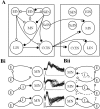
The analysis of synaptic properties in neural networks has focused on the properties of individual synapses. As a result, little is known of how neural assemblies arise from the connectivity and functional properties of different classes of network neurons. I examined synaptic properties in the lamprey locomotor network. Here I show that, in addition to their monosynaptic inputs to motor neurons, a proportion of the excitatory network interneurons (EINs) evoke an activity-dependent disynaptic feedforward inhibitory input. Connections from the excitatory interneurons to small ipsilateral inhibitory interneurons were found that could account for the feedforward inhibition. Both synapses in the disynaptic pathway exhibited activity-dependent facilitation during physiologically relevant spike trains, which could contribute to the delayed, activity-dependent development of the feedforward IPSP. Although it was not as common as the feedforward inhibition, the excitatory interneurons could also evoke feedforward excitatory inputs in motor neurons. EIN inputs to motor neurons usually depress during spike trains. In connections in which a delayed IPSP occurred, blocking the feedforward inhibition in motor neurons or preventing the activation of the disynaptic pathway abolished the depression of the direct EPSP during the spike train and could reveal an underlying facilitation. The feedforward inhibition thus heterosynaptically depressed the direct excitatory input to motor neurons. Activity-dependent heterosynaptic effects acting within network cellular assemblies can thus influence the integration of synaptic inputs in motor neurons. This could help to terminate ipsilateral motor neuron spiking during network activity.
Figures

References
-
- Alford S, Grillner S ( 1990) CNQX and DNQX block non-NMDA synaptic transmission but not NMDA-evoked locomotion in lamprey spinal cord. Brain Res 506: 297-302. - PubMed
-
- Berry M, Pentreath V ( 1976) Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res 105: 1-20. - PubMed
-
- Buchanan J ( 1993) Electrophysiological properties of identified classes of lamprey spinal neurons. J Neurophysiol 70: 2313-2325. - PubMed
-
- Buchanan J ( 1999) Commissural interneurons in rhythm generation and intersegmental coupling in the lamprey spinal cord. J Neurophysiol 81: 2037-2045. - PubMed
-
- Buchanan J ( 2001) Contributions of identifiable neurons and neuron classes to lamprey vertebrate neurobiology. Prog Neurobiol 63: 441-466. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
