Analgesia induced by dietary restriction is mediated by the kappa-opioid system
- PMID: 14657170
- PMCID: PMC6741040
- DOI: 10.1523/JNEUROSCI.23-35-11120.2003
Analgesia induced by dietary restriction is mediated by the kappa-opioid system
Abstract
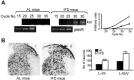
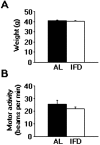
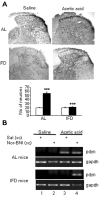
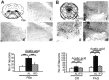
Progress in the control and treatment of pain may be facilitated by a better understanding of mechanisms underlying nociceptive processing. Here we show that mice subjected to an intermittent fasting diet (IFD) display markedly reduced responses in models of thermal and visceral pain compared with mice fed ad libitum (AL). Pharmacological analyses suggest that a change in the endogenous kappa-opioid system underlies IFD-induced analgesia. The levels of prodynorphin mRNA and kappa-opioid receptors in the spinal cord are higher in IFD than in AL mice. Furthermore, in spinal cord nuclear protein extracts, the activity of the transcriptional repressor DREAM (downstream regulatory element antagonist modulator), the main regulator of prodynorphin expression, is lower in IFD than in AL mice. Finally, c-Fos expression in dorsal spinal cord after noxious stimulation is significantly lower in IFD than in AL animals, indicating that dynorphin could block nociceptive information at the spinal cord. These results suggest that dietary restriction together with administration of kappa-opioid agonists could be useful as a new therapeutic approach for pain relief.
Figures


Similar articles
-
Functional blockage of the cannabinoid receptor type 1 evokes a kappa-opiate-dependent analgesia.J Neurochem. 2007 Dec;103(6):2629-39. doi: 10.1111/j.1471-4159.2007.05000.x. J Neurochem. 2007. PMID: 17953671
-
Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance.J Neurosci. 2004 May 12;24(19):4576-84. doi: 10.1523/JNEUROSCI.5552-03.2004. J Neurosci. 2004. PMID: 15140929 Free PMC article.
-
Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses.J Neurosci. 2003 Jul 2;23(13):5674-83. doi: 10.1523/JNEUROSCI.23-13-05674.2003. J Neurosci. 2003. PMID: 12843270 Free PMC article.
-
Opioids in chronic pain.Eur J Pharmacol. 2001 Oct 19;429(1-3):79-91. doi: 10.1016/s0014-2999(01)01308-5. Eur J Pharmacol. 2001. PMID: 11698029 Review.
-
When the DREAM is gone: from basic science to future prospectives in pain management and beyond.Expert Opin Ther Targets. 2003 Apr;7(2):249-63. doi: 10.1517/14728222.7.2.249. Expert Opin Ther Targets. 2003. PMID: 12667101 Review.
Cited by
-
Inhibitory effect of lidocaine on pain and itch using formalin-induced nociception and 5'-guanidinonaltrindole-induced scratching models in mice: behavioral and neuroanatomical evidence.Eur J Pharmacol. 2009 Aug 15;616(1-3):141-6. doi: 10.1016/j.ejphar.2009.06.026. Epub 2009 Jun 21. Eur J Pharmacol. 2009. PMID: 19549515 Free PMC article.
-
Effect of cholera toxin administered supraspinally or spinally on the blood glucose level in pain and d-glucose fed animal models.Korean J Physiol Pharmacol. 2013 Apr;17(2):163-7. doi: 10.4196/kjpp.2013.17.2.163. Epub 2013 Apr 10. Korean J Physiol Pharmacol. 2013. PMID: 23626479 Free PMC article.
-
Postnatal proteasome inhibition induces neurodegeneration and cognitive deficiencies in adult mice: a new model of neurodevelopment syndrome.PLoS One. 2011;6(12):e28927. doi: 10.1371/journal.pone.0028927. Epub 2011 Dec 12. PLoS One. 2011. PMID: 22174927 Free PMC article.
-
Cholinergic septo-hippocampal innervation is required for trace eyeblink classical conditioning.Learn Mem. 2005 Nov-Dec;12(6):557-63. doi: 10.1101/lm.28105. Epub 2005 Nov 14. Learn Mem. 2005. PMID: 16287719 Free PMC article.
-
Antinociceptive effects of caloric restriction on post-incisional pain in nonobese rats.Sci Rep. 2017 May 11;7(1):1805. doi: 10.1038/s41598-017-01909-8. Sci Rep. 2017. PMID: 28496116 Free PMC article.
References
-
- Akil H, Meng F, Mansour A, Thompson R, Xie GX, Watson S ( 1996) Cloning and characterization of multiple opioid receptors. NIDA Res Monogr 161: 127-140. - PubMed
-
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP ( 2003) Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA 100: 2616-2620. - PMC - PubMed
-
- Brochu M, Poehlman ET, Ades PA ( 2000) Obesity, body fat distribution, and coronary artery disease. J Cardiopulm Rehabil 20: 96-108. - PubMed
-
- Campos D, Jiménez-Díaz L, Naranjo JR, Carrión AM ( 2003) Ca-dependent prodynorphin transcriptional derepression in neuroblastoma cells is exerted through DREAM protein activity in a kinase-independent manner. Mol Cell Neurosci 22: 135-145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
