Physiology of cells in the central lobes of the mormyrid cerebellum
- PMID: 14657174
- PMCID: PMC6741054
- DOI: 10.1523/JNEUROSCI.23-35-11147.2003
Physiology of cells in the central lobes of the mormyrid cerebellum
Abstract
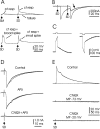
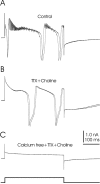
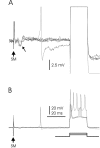
The cerebellum of mormyrid electric fish is unusual for its size and for the regularity of its histology. The circuitry of the mormyrid cerebellum is also different from that of the mammalian cerebellum in that mormyrid Purkinje cell axons terminate locally within the cortex on efferent cells, and the cellular regions of termination for climbing fibers and parallel fibers are well separated. These and other features suggest that the mormyrid cerebellum may be a useful site for addressing some functional issues regarding cerebellar circuitry. We have therefore begun to examine the physiology of the mormyrid cerebellum by recording intracellularly from morphologically identified Purkinje cells, efferent cells, Golgi cells, and stellate cells in in vitro slices. Mormyrid Purkinje cells respond to parallel fiber input with an AMPA-mediated EPSP that shows paired pulse facilitation and to climbing fiber input with a large all-or-none AMPA-mediated EPSP that shows paired pulse depression. Recordings from the somas of Purkinje cells show three types of spikes in response to injected current: a small, narrow sodium spike; a large, broad sodium spike; and a large broad calcium spike. Efferent cells, Golgi cells, and stellate cells respond to parallel fiber input with an EPSP or EPSP-IPSP sequence and show only large, narrow spikes in response to intracellular current injection. We conclude that the physiology of the mormyrid cerebellum is similar in many ways to the mammalian cerebellum but is also different in ways that may prove instructive concerning the functional circuitry of the cerebellum.
Figures








Similar articles
-
Electrophysiological characteristics of cells in the anterior caudal lobe of the mormyrid cerebellum.Neuroscience. 2010 Nov 24;171(1):79-91. doi: 10.1016/j.neuroscience.2010.08.033. Epub 2010 Aug 21. Neuroscience. 2010. PMID: 20732390
-
Functional circuitry of a unique cerebellar specialization: the valvula cerebelli of a mormyrid fish.Neuroscience. 2011 May 19;182:11-31. doi: 10.1016/j.neuroscience.2011.03.014. Epub 2011 Mar 21. Neuroscience. 2011. PMID: 21414387
-
Cell morphology and circuitry in the central lobes of the mormyrid cerebellum.J Comp Neurol. 2006 Jul 20;497(3):309-25. doi: 10.1002/cne.20983. J Comp Neurol. 2006. PMID: 16736465
-
Physiology of electrosensory lateral line lobe neurons in Gnathonemus petersii.J Exp Biol. 1999 May;202(Pt 10):1301-9. doi: 10.1242/jeb.202.10.1301. J Exp Biol. 1999. PMID: 10210670 Review.
-
Climbing fibers mediate vestibular modulation of both "complex" and "simple spikes" in Purkinje cells.Cerebellum. 2015 Oct;14(5):597-612. doi: 10.1007/s12311-015-0725-1. Cerebellum. 2015. PMID: 26424151 Review.
Cited by
-
Integration of Swimming-Related Synaptic Excitation and Inhibition by olig2+ Eurydendroid Neurons in Larval Zebrafish Cerebellum.J Neurosci. 2020 Apr 8;40(15):3063-3074. doi: 10.1523/JNEUROSCI.2322-19.2020. Epub 2020 Mar 5. J Neurosci. 2020. PMID: 32139583 Free PMC article.
-
Distinct responses of Purkinje neurons and roles of simple spikes during associative motor learning in larval zebrafish.Elife. 2017 May 25;6:e22537. doi: 10.7554/eLife.22537. Elife. 2017. PMID: 28541889 Free PMC article.
-
Development, circuitry, and function of the zebrafish cerebellum.Cell Mol Life Sci. 2023 Jul 25;80(8):227. doi: 10.1007/s00018-023-04879-5. Cell Mol Life Sci. 2023. PMID: 37490159 Free PMC article. Review.
-
Synaptic variance and action potential firing of cerebellar output neurons during motor learning in larval zebrafish.Curr Biol. 2023 Aug 21;33(16):3299-3311.e3. doi: 10.1016/j.cub.2023.06.045. Epub 2023 Jul 7. Curr Biol. 2023. PMID: 37421952 Free PMC article.
-
Distribution and function of potassium channels in the electrosensory lateral line lobe of weakly electric apteronotid fish.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006 Jun;192(6):637-48. doi: 10.1007/s00359-006-0103-z. Epub 2006 Jan 20. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006. PMID: 16425062 Review.
References
-
- Aghajanian GK, Rasmussen K ( 1989) Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse 3: 331-338. - PubMed
-
- Bell CC, Han VZ, Sugawara S, Grant K ( 1997) Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature 387: 278-281. - PubMed
-
- Eccles J, Ito M, Szentagothai J ( 1967) The cerebellum as a neuronal machine. Berlin: Springer.
-
- Fox CA ( 1962) The structure of the cerebellar cortex. In: Correlative anatomy of the nervous system (Crosby EC, Humphrey TH, Lauer EW, eds), pp 193-198. New York: Macmillan.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
