Neuronal avalanches in neocortical circuits
- PMID: 14657176
- PMCID: PMC6741045
- DOI: 10.1523/JNEUROSCI.23-35-11167.2003
Neuronal avalanches in neocortical circuits
Abstract
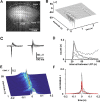
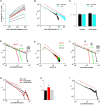
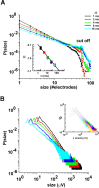
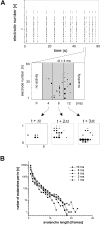
Networks of living neurons exhibit diverse patterns of activity, including oscillations, synchrony, and waves. Recent work in physics has shown yet another mode of activity in systems composed of many nonlinear units interacting locally. For example, avalanches, earthquakes, and forest fires all propagate in systems organized into a critical state in which event sizes show no characteristic scale and are described by power laws. We hypothesized that a similar mode of activity with complex emergent properties could exist in networks of cortical neurons. We investigated this issue in mature organotypic cultures and acute slices of rat cortex by recording spontaneous local field potentials continuously using a 60 channel multielectrode array. Here, we show that propagation of spontaneous activity in cortical networks is described by equations that govern avalanches. As predicted by theory for a critical branching process, the propagation obeys a power law with an exponent of -3/2 for event sizes, with a branching parameter close to the critical value of 1. Simulations show that a branching parameter at this value optimizes information transmission in feedforward networks, while preventing runaway network excitation. Our findings suggest that "neuronal avalanches" may be a generic property of cortical networks, and represent a mode of activity that differs profoundly from oscillatory, synchronized, or wave-like network states. In the critical state, the network may satisfy the competing demands of information transmission and network stability.
Figures



References
-
- Abeles M ( 1992) Corticonics. New York: Cambridge UP.
-
- Aertsen A, Diesmann M, Gewaltig MO ( 1996) Propagation of synchronous spiking activity in feedforward neural networks. J Physiol (Paris) 90: 243-247. - PubMed
-
- Arieli A ( 1992) Novel strategies to unravel mechanisms of cortical function: from macro- to micro-electrophysiological recordings. In: Information processing in the cortex (Aertsen A, Braitenberg V, eds), pp 123-137. New York: Springer.
-
- Bak P, Tang C, Wiesenfeld K ( 1987) Self-organized criticality: an explanation of the 1/f noise. Phys Rev Lett 59: 381-384. - PubMed
-
- Bove M, Genta G, Verreschi G, Grattarola M ( 1996) Characterization and interpretation of electrical signals from random networks of cultured neurons. Technol Health Care 4: 77-86. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
