Dopamine reduces odor- and elevated-K(+)-induced calcium responses in mouse olfactory receptor neurons in situ
- PMID: 14657189
- PMCID: PMC2955887
- DOI: 10.1152/jn.00670.2003
Dopamine reduces odor- and elevated-K(+)-induced calcium responses in mouse olfactory receptor neurons in situ
Abstract
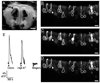
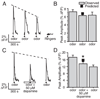
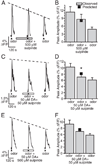
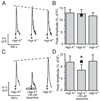
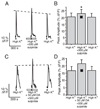
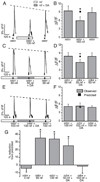
Although D2 dopamine receptors have been localized to olfactory receptor neurons (ORNs) and dopamine has been shown to modulate voltage-gated ion channels in ORNs, dopaminergic modulation of either odor responses or excitability in mammalian ORNs has not previously been demonstrated. We found that <50 microM dopamine reversibly suppresses odor-induced Ca2+ transients in ORNs. Confocal laser imaging of 300-microm-thick slices of neonatal mouse olfactory epithelium loaded with the Ca(2+)-indicator dye fluo-4 AM revealed that dopaminergic suppression of odor responses could be blocked by the D2 dopamine receptor antagonist sulpiride (<500 microM). The dopamine-induced suppression of odor responses was completely reversed by 100 microM nifedipine, suggesting that D2 receptor activation leads to an inhibition of L-type Ca2+ channels in ORNs. In addition, dopamine reversibly reduced ORN excitability as evidenced by reduced amplitude and frequency of Ca2+ transients in response to elevated K(+), which activates voltage-gated Ca2+ channels in ORNs. As with the suppression of odor responses, the effects of dopamine on ORN excitability were blocked by the D2 dopamine receptor antagonist sulpiride (<500 microM). The observation of dopaminergic modulation of odor-induced Ca2+ transients in ORNs adds to the growing body of work showing that olfactory receptor neurons can be modulated at the periphery. Dopamine concentrations in nasal mucus increase in response to noxious stimuli, and thus D2 receptor-mediated suppression of voltage-gated Ca2+ channels may be a novel neuroprotective mechanism for ORNs.
Figures

Similar articles
-
T-type Ca2+ channels mediate propagation of odor-induced Ca2+ transients in rat olfactory receptor neurons.Neuroscience. 2007 Jan 19;144(2):702-13. doi: 10.1016/j.neuroscience.2006.10.012. Epub 2006 Nov 15. Neuroscience. 2007. PMID: 17110049
-
Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics.J Neurosci. 1997 Jun 1;17(11):4136-48. doi: 10.1523/JNEUROSCI.17-11-04136.1997. J Neurosci. 1997. PMID: 9151731 Free PMC article.
-
T-type Ca2+ channels contribute to IBMX/forskolin- and K(+)-induced Ca(2+) transients in porcine olfactory receptor neurons.Neurosci Res. 2007 Jan;57(1):129-39. doi: 10.1016/j.neures.2006.09.016. Epub 2006 Oct 30. Neurosci Res. 2007. PMID: 17074407
-
Intensity of odorant stimulation affects mode of Ca2+ dynamics in rat olfactory receptor neurons.Neurosci Res. 2006 Aug;55(4):410-20. doi: 10.1016/j.neures.2006.04.012. Epub 2006 May 30. Neurosci Res. 2006. PMID: 16730825
-
Activation of purinergic receptor subtypes modulates odor sensitivity.J Neurosci. 2003 Sep 10;23(23):8291-301. doi: 10.1523/JNEUROSCI.23-23-08291.2003. J Neurosci. 2003. PMID: 12967991 Free PMC article.
Cited by
-
Increases in intracellular calcium via activation of potentially multiple phospholipase C isozymes in mouse olfactory neurons.Front Cell Neurosci. 2014 Oct 21;8:336. doi: 10.3389/fncel.2014.00336. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25374507 Free PMC article.
-
Olfactory receptor neuron dysfunction in schizophrenia.Neuropsychopharmacology. 2009 Feb;34(3):767-74. doi: 10.1038/npp.2008.139. Epub 2008 Aug 27. Neuropsychopharmacology. 2009. PMID: 18754006 Free PMC article.
-
The β2-adrenergic receptor as a surrogate odorant receptor in mouse olfactory sensory neurons.Mol Cell Neurosci. 2014 Jan;58:1-10. doi: 10.1016/j.mcn.2013.10.010. Epub 2013 Nov 6. Mol Cell Neurosci. 2014. PMID: 24211702 Free PMC article.
-
Cannabinoid action in the olfactory epithelium.Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2967-72. doi: 10.1073/pnas.0609067104. Epub 2007 Feb 14. Proc Natl Acad Sci U S A. 2007. PMID: 17301239 Free PMC article.
-
Neuroleptic-induced parkinsonism is associated with olfactory dysfunction.J Neurol. 2008 Oct;255(10):1574-9. doi: 10.1007/s00415-008-0993-5. Epub 2008 Sep 3. J Neurol. 2008. PMID: 18769856
References
-
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. - PubMed
-
- Berkowicz DA, Trombley PQ. Dopaminergic modulation at the olfactory nerve synapse. Brain Res. 2000;855:90–99. - PubMed
-
- Chen Y, Getchell TV, Sparks DL, Getchell ML. Patterns of adrenergic and peptidergic innervation in human olfactory mucosa: age-related trends. J Comp Neurol. 1993;334:104–116. - PubMed
-
- Coronas V, Feron F, Hen R, Sicard G, Jourdan F, Moyse E. In vitro induction of apoptosis or differentiation by dopamine in an immortalized olfactory neuronal cell line. J Neurochem. 1997a;69:1870–1881. - PubMed
-
- Coronas V, Krantic S, Jourdan F, Moyse E. Dopamine receptor coupling to adenylyl cyclase in rat olfactory pathway: a combined pharmacological-radioautographic approach. Neuroscience. 1999;90:69–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

