An immunologically privileged retinal antigen elicits tolerance: major role for central selection mechanisms
- PMID: 14657219
- PMCID: PMC2194140
- DOI: 10.1084/jem.20030413
An immunologically privileged retinal antigen elicits tolerance: major role for central selection mechanisms
Abstract
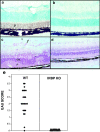
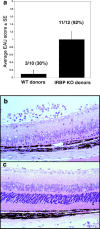
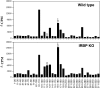
Immunologically privileged retinal antigens can serve as targets of experimental autoimmune uveitis (EAU), a model for human uveitis. The tolerance status of susceptible strains, whose target antigen is not expressed in the thymus at detectable levels, is unclear. Here, we address this issue directly by analyzing the consequences of genetic deficiency versus sufficiency of a uveitogenic retinal antigen, interphotoreceptor retinoid-binding protein (IRBP). IRBP-knockout (KO) and wild-type (WT) mice on a highly EAU-susceptible background were challenged with IRBP. The KO mice had greatly elevated responses to IRBP, an altered recognition of IRBP epitopes, and their primed T cells induced exacerbated disease in WT recipients. Ultrasensitive immunohistochemical staining visualized sparse IRBP-positive cells, undetectable by conventional assays, in thymi of WT (but not of KO) mice. IRBP message was PCR amplified from these cells after microdissection. Thymus transplantation between KO and WT hosts demonstrated that this level of expression is functionally relevant and sets the threshold of immune (and autoimmune) reactivity. Namely, KO recipients of WT thymi generated reduced IRBP-specific responses, and WT recipients of KO thymi developed enhanced responses and a highly exacerbated disease. Repertoire culling and thymus-dependent CD25+ T cells were implicated in this effect. Thus, uveitis-susceptible individuals display a detectable and functionally significant tolerance to their target antigen, in which central mechanisms play a prominent role.
Figures
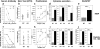



References
-
- Caspi, R.R. 1999. Immune mechanisms in uveitis. Springer Semin. Immunopathol. 21:113–124. - PubMed
-
- Gery, I., and J.W. Streilein. 1994. Autoimmunity in the eye and its regulation. Curr. Opin. Immunol. 6:938–945. - PubMed
-
- Caspi, R.R., C.C. Chan, B. Wiggert, and G.J. Chader. 1990. The mouse as a model of experimental autoimmune uveoretinitis (EAU). Curr. Eye Res. 9(Suppl.):169–174. - PubMed
-
- Caspi, R.R., F.G. Roberge, C.C. Chan, B. Wiggert, G.J. Chader, L.A. Rozenszajn, Z. Lando, and R.B. Nussenblatt. 1988. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol. 140:1490–1495. - PubMed
-
- Streilein, J.W., S. Masli, M. Takeuchi, and T. Kezuka. 2002. The eye's view of antigen presentation. Hum. Immunol. 63:435–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

