Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor
- PMID: 14657220
- PMCID: PMC2194130
- DOI: 10.1084/jem.20030932
Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor
Abstract
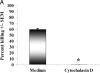
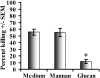
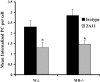
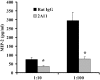
Innate immune mechanisms against Pneumocystis carinii, a frequent cause of pneumonia in immunocompromised individuals, are not well understood. Using both real time polymerase chain reaction as a measure of organism viability and fluorescent deconvolution microscopy, we show that nonopsonic phagocytosis of P. carinii by alveolar macrophages is mediated by the Dectin-1 beta-glucan receptor and that the subsequent generation of hydrogen peroxide is involved in alveolar macrophage-mediated killing of P. carinii. The macrophage Dectin-1 beta-glucan receptor colocalized with the P. carinii cyst wall. However, blockage of Dectin-1 with high concentrations of anti-Dectin-1 antibody inhibited binding and concomitant killing of P. carinii by alveolar macrophages. Furthermore, RAW 264.7 macrophages overexpressing Dectin-1 bound P. carinii at a higher level than control RAW cells. In the presence of Dectin-1 blockage, killing of opsonized P. carinii could be restored through FcgammaRII/III receptors. Opsonized P. carinii could also be efficiently killed in the presence of FcgammaRII/III receptor blockage through Dectin-1-mediated phagocytosis. We further show that Dectin-1 is required for P. carinii-induced macrophage inflammatory protein 2 production by alveolar macrophages. Taken together, these results show that nonopsonic phagocytosis and subsequent killing of P. carinii by alveolar macrophages is dependent upon recognition by the Dectin-1 beta-glucan receptor.
Figures
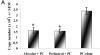
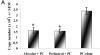










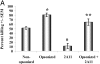
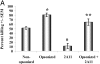
References
-
- Ives, N.J., B.G. Gazzard, and P.J. Easterbrook. 2001. The changing pattern of AIDS-defining illnesses with the introduction of highly active antiretroviral therapy (HAART) in a London clinic. J. Infect. 42:134–139. - PubMed
-
- Morris, A., R.M. Wachter, J. Luce, J. Turner, and L. Huang. 2003. Improved survival with highly active antiretroviral therapy in HIV-infected patients with severe Pneumocystis carinii pneumonia. AIDS. 17:73–80. - PubMed
-
- Sullivan, J.H., R.D. Moore, J.C. Keruly, and R.E. Chaisson. 2000. Effect of antiretroviral therapy on the incidence of bacterial pneumonia in patients with advanced HIV infection. Am. J. Respir. Crit. Care Med. 162:64–67. - PubMed
-
- Mayaud, C., A. Parrot, and J. Cadranel. 2002. Pyogenic bacterial lower respiratory tract infection in human immunodeficiency virus-infected patients. Eur. Respir. J. Suppl. 36:28s–39s. - PubMed
-
- Vassallo, R., C.F. Thomas, Jr., Z. Vuk-Pavlovic, and A.H. Limper. 1999. Alveolar macrophage interactions with Pneumocystis carinii. J. Lab. Clin. Med. 133:535–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

