GDNF-deprived sympathetic neurons die via a novel nonmitochondrial pathway
- PMID: 14657232
- PMCID: PMC2173604
- DOI: 10.1083/jcb.200305083
GDNF-deprived sympathetic neurons die via a novel nonmitochondrial pathway
Abstract
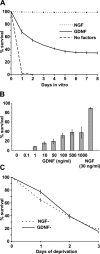
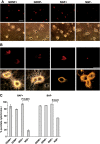
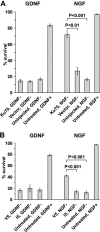
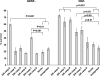
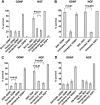
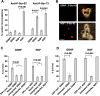
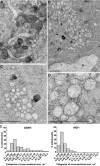
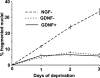
The mitochondrial death pathway is triggered in cultured sympathetic neurons by deprivation of nerve growth factor (NGF), but the death mechanisms activated by deprivation of other neurotrophic factors are poorly studied. We compared sympathetic neurons deprived of NGF to those deprived of glial cell line-derived neurotrophic factor (GDNF). In contrast to NGF-deprived neurons, GDNF-deprived neurons did not die via the mitochondrial pathway. Indeed, cytochrome c was not released to the cytosol; Bax and caspase-9 and -3 were not involved; overexpressed Bcl-xL did not block the death; and the mitochondrial ultrastructure was not changed. Similarly to NGF-deprived neurons, the death induced by GDNF removal is associated with increased autophagy and requires multiple lineage kinases, c-Jun and caspase-2 and -7. Serine 73 of c-Jun was phosphorylated in both NGF- and GDNF-deprived neurons, whereas serine 63 was phosphorylated only in NGF-deprived neurons. In many NGF-deprived neurons, the ultrastructure of the mitochondria was changed. Thus, a novel nonmitochondrial caspase-dependent death pathway is activated in GDNF-deprived sympathetic neurons.
Figures

References
-
- Airaksinen, M.S., and M. Saarma. 2002. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3:383–394. - PubMed
-
- Besirli, C.G., and E.M. Johnson, Jr. 2003. JNK-independent activation of c-Jun during neuronal apoptosis Induced by multiple DNA-damaging agents. J. Biol. Chem. 278:22357–22366. - PubMed
-
- Clarke, P.G. 1990. Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol. (Berl.). 181:195–213. - PubMed
-
- Deckwerth, T.L., J.L. Elliott, C.M. Knudson, E.M. Johnson, Jr., W.D. Snider, and S.J. Korsmeyer. 1996. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 17:401–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

