Aberrant myofibril assembly in tropomodulin1 null mice leads to aborted heart development and embryonic lethality
- PMID: 14657235
- PMCID: PMC2173615
- DOI: 10.1083/jcb.200308164
Aberrant myofibril assembly in tropomodulin1 null mice leads to aborted heart development and embryonic lethality
Abstract
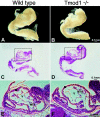
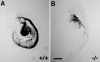
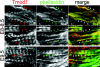
Tropomodulin1 (Tmod1) caps thin filament pointed ends in striated muscle, where it controls filament lengths by regulating actin dynamics. Here, we investigated myofibril assembly and heart development in a Tmod1 knockout mouse. In the absence of Tmod1, embryonic development appeared normal up to embryonic day (E) 8.5. By E9.5, heart defects were evident, including aborted development of the myocardium and inability to pump, leading to embryonic lethality by E10.5. Confocal microscopy of hearts of E8-8.5 Tmod1 null embryos revealed structures resembling nascent myofibrils with continuous F-actin staining and periodic dots of alpha-actinin, indicating that I-Z-I complexes assembled in the absence of Tmod1. Myomesin, a thick filament component, was also assembled normally along these structures, indicating that thick filament assembly is independent of Tmod1. However, myofibrils did not become striated, and gaps in F-actin staining (H zones) were never observed. We conclude that Tmod1 is required for regulation of actin filament lengths and myofibril maturation; this is critical for heart morphogenesis during embryonic development.
Figures



Similar articles
-
Mechanisms of thin filament assembly in embryonic chick cardiac myocytes: tropomodulin requires tropomyosin for assembly.J Cell Biol. 1995 May;129(3):683-95. doi: 10.1083/jcb.129.3.683. J Cell Biol. 1995. PMID: 7730404 Free PMC article.
-
Disruption in the tropomodulin1 (Tmod1) gene compromises cardiomyocyte development in murine embryonic stem cells by arresting myofibril maturation.Dev Biol. 2005 Jun 15;282(2):336-48. doi: 10.1016/j.ydbio.2005.03.015. Dev Biol. 2005. PMID: 15950601
-
Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils.J Cell Sci. 1999 Apr;112 ( Pt 8):1111-23. doi: 10.1242/jcs.112.8.1111. J Cell Sci. 1999. PMID: 10085247
-
Capping actin filament growth: tropomodulin in muscle and nonmuscle cells.Soc Gen Physiol Ser. 1997;52:79-89. Soc Gen Physiol Ser. 1997. PMID: 9210222 Review.
-
Defining actin filament length in striated muscle: rulers and caps or dynamic stability?Annu Rev Cell Dev Biol. 1998;14:487-525. doi: 10.1146/annurev.cellbio.14.1.487. Annu Rev Cell Dev Biol. 1998. PMID: 9891791 Review.
Cited by
-
Cypher and Enigma homolog protein are essential for cardiac development and embryonic survival.J Am Heart Assoc. 2015 May 5;4(5):e001950. doi: 10.1161/JAHA.115.001950. J Am Heart Assoc. 2015. PMID: 25944877 Free PMC article.
-
Actin-associated proteins and cardiomyopathy-the 'unknown' beyond troponin and tropomyosin.Biophys Rev. 2018 Aug;10(4):1121-1128. doi: 10.1007/s12551-018-0428-1. Epub 2018 Jun 5. Biophys Rev. 2018. PMID: 29869751 Free PMC article. Review.
-
Phosphorylation of tropomodulin1 contributes to the regulation of actin filament architecture in cardiac muscle.FASEB J. 2014 Sep;28(9):3987-95. doi: 10.1096/fj.13-246009. Epub 2014 Jun 2. FASEB J. 2014. PMID: 24891520 Free PMC article.
-
Transgenic Expression of the Formin Protein Fhod3 Selectively in the Embryonic Heart: Role of Actin-Binding Activity of Fhod3 and Its Sarcomeric Localization during Myofibrillogenesis.PLoS One. 2016 Feb 5;11(2):e0148472. doi: 10.1371/journal.pone.0148472. eCollection 2016. PLoS One. 2016. PMID: 26848968 Free PMC article.
-
The cardiomyopathy-associated K15N mutation in tropomyosin alters actin filament pointed end dynamics.Arch Biochem Biophys. 2017 Sep 15;630:18-26. doi: 10.1016/j.abb.2017.07.006. Epub 2017 Jul 18. Arch Biochem Biophys. 2017. PMID: 28732641 Free PMC article.
References
-
- Almenar-Queralt, A., C.C. Gregorio, and V.M. Fowler. 1999. a. Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J. Cell Sci. 112:1111–1123. - PubMed
-
- Almenar-Queralt, A., A. Lee, C.A. Conley, L. Ribas de Pouplana, and V.M. Fowler. 1999. b. Identification of a novel tropomodulin isoform, skeletal tropomodulin, that caps actin filament pointed ends in fast skeletal muscle. J. Biol. Chem. 274:28466–28475. - PubMed
-
- Auerbach, D., B. Rothen-Rutishauser, S. Bantle, M. Leu, E. Ehler, D. Helfman, and J.-C. Perriard. 1997. Molecular mechanisms of myofibril assembly in the heart. Cell Struct. Funct. 22:139–146. - PubMed
-
- Bamburg, J.R., A. McGough, and S. Ono. 1999. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 9:364–370. - PubMed
-
- Ben-Arie, H.N., H.J. Bellen, D.L. Armstrong, A.E. McCall, P.R. Gordadze, Q. Guo, M.M. Matzuk, and H.Y. Zoghbi. 1997. Math1 is essential for genesis of cerebellar granule neurons. Nature. 390:169–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

