Translocation of FGF-1 and FGF-2 across vesicular membranes occurs during G1-phase by a common mechanism
- PMID: 14657241
- PMCID: PMC329394
- DOI: 10.1091/mbc.e03-08-0589
Translocation of FGF-1 and FGF-2 across vesicular membranes occurs during G1-phase by a common mechanism
Abstract
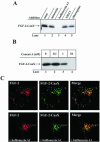
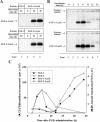
The entry of exogenous fibroblast growth factor 2 (FGF-2) to the cytosolic/nuclear compartment was studied and compared with the translocation mechanism used by FGF-1. To differentiate between external and endogenous growth factor, we used FGF-2 modified to contain a farnesylation signal, a CaaX-box. Because farnesylation occurs only in the cytosol and nucleoplasm, farnesylation of exogenous FGF-2-CaaX was taken as evidence that the growth factor had translocated across cellular membranes. We found that FGF-2 translocation occurred in endothelial cells and fibroblasts, which express FGF receptors, and that the efficiency of translocation was increased in the presence of heparin. Concomitantly with translocation, the 18-kDa FGF-2 was N-terminally cleaved to yield a 16-kDa form. Translocation of FGF-2 required PI3-kinase activity but not transport through the Golgi apparatus. Inhibition of endosomal acidification did not prevent translocation, whereas dissipation of the vesicular membrane potential completely blocked it. The data indicate that translocation occurs from intracellular vesicles containing proton pumps and that an electrical potential across the vesicle membrane is required. Translocation of both FGF-1 and FGF-2 occurred during most of G(1) but decreased shortly before the G(1)-->S transition. A common mechanism for FGF-1 and FGF-2 translocation into cells is postulated.
Figures


Similar articles
-
Vesicle transmembrane potential is required for translocation to the cytosol of externally added FGF-1.EMBO J. 2002 Sep 2;21(17):4480-90. doi: 10.1093/emboj/cdf472. EMBO J. 2002. PMID: 12198150 Free PMC article.
-
Perinuclear localization of an intracellular binding protein related to the fibroblast growth factor (FGF) receptor 1 is temporally associated with the nuclear trafficking of FGF-2 in proliferating epiphyseal growth plate chondrocytes.Endocrinology. 1996 Nov;137(11):5078-89. doi: 10.1210/endo.137.11.8895382. Endocrinology. 1996. PMID: 8895382
-
FGF-1 and FGF-2 require the cytosolic chaperone Hsp90 for translocation into the cytosol and the cell nucleus.J Biol Chem. 2006 Apr 21;281(16):11405-12. doi: 10.1074/jbc.M600477200. Epub 2006 Feb 22. J Biol Chem. 2006. PMID: 16495214
-
Characterization of a non-tyrosine kinase FGF-binding protein.Ann N Y Acad Sci. 1991;638:195-203. doi: 10.1111/j.1749-6632.1991.tb49030.x. Ann N Y Acad Sci. 1991. PMID: 1723853 Review. No abstract available.
-
Unconventional secretion mediated by direct protein self-translocation across the plasma membranes of mammalian cells.Trends Biochem Sci. 2022 Aug;47(8):699-709. doi: 10.1016/j.tibs.2022.04.001. Epub 2022 Apr 27. Trends Biochem Sci. 2022. PMID: 35490075 Review.
Cited by
-
Identification of new FGF1 binding partners-Implications for its intracellular function.IUBMB Life. 2016 Mar;68(3):242-51. doi: 10.1002/iub.1480. Epub 2016 Feb 2. IUBMB Life. 2016. PMID: 26840910 Free PMC article.
-
Cytotoxic Conjugates of Fibroblast Growth Factor 2 (FGF2) with Monomethyl Auristatin E for Effective Killing of Cells Expressing FGF Receptors.ACS Omega. 2017 Jul 31;2(7):3792-3805. doi: 10.1021/acsomega.7b00116. Epub 2017 Jul 21. ACS Omega. 2017. PMID: 30023704 Free PMC article.
-
Phosphorylation-regulated nucleocytoplasmic trafficking of internalized fibroblast growth factor-1.Mol Biol Cell. 2005 Feb;16(2):794-810. doi: 10.1091/mbc.e04-05-0389. Epub 2004 Dec 1. Mol Biol Cell. 2005. PMID: 15574884 Free PMC article.
-
Nuclear Localization Sequence of FGF1 Is Not Required for Its Intracellular Anti-Apoptotic Activity in Differentiated Cells.Cells. 2022 Feb 2;11(3):522. doi: 10.3390/cells11030522. Cells. 2022. PMID: 35159330 Free PMC article.
-
Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system.PLoS Pathog. 2007 Mar;3(3):e51. doi: 10.1371/journal.ppat.0030051. PLoS Pathog. 2007. PMID: 17397264 Free PMC article.
References
-
- Arnaud, E., Touriol, C., Boutonnet, C., Gensac, M.C., Vagner, S., Prats, H., and Prats, A.C. (1999). A new 34-kilodalton isoform of human fibroblast growth factor 2 is cap dependently synthesized by using a non-AUG start codon and behaves as a survival factor. Mol. Cell. Biol. 19, 505-514. - PMC - PubMed
-
- Bailly, K., Soulet, F., Leroy, D., Amalric, F., and Bouche, G. (2000). Uncoupling of cell proliferation and differentiation activities of basic fibroblast growth factor. FASEB J. 14, 333-344. - PubMed
-
- Bomsel, M., Parton, R., Kuznetsov, S.A., Schroer, T.A., and Gruenberg, J. (1990). Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell 62, 719-731. - PubMed
-
- Bonnet, H., Filhol, O., Truchet, I., Brethenou, P., Cochet, C., Amalric, F., and Bouche, G. (1996). Fibroblast growth factor-2 binds to the regulatory beta subunit of CK2 and directly stimulates CK2 activity toward nucleolin. J. Biol. Chem. 271, 24781-24787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

