Clustering induces a lateral redistribution of alpha 2 beta 1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization
- PMID: 14657242
- PMCID: PMC329276
- DOI: 10.1091/mbc.e03-08-0588
Clustering induces a lateral redistribution of alpha 2 beta 1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization
Abstract
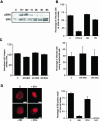
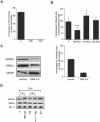
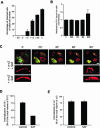
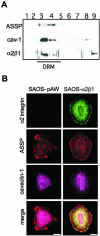
Integrin alpha 2 beta 1 mediates the binding of several epithelial and mesenchymal cell types to collagen. The composition of the surrounding plasma membrane, especially caveolin-1- and cholesterol-containing membrane structures called caveolae, may be important to integrin signaling. On cell surface alpha 2 beta 1 integrin was located in the raft like membrane domain, rich in GPI-anchored proteins, rather than in caveolae. However, when antibodies were used to generate clusters of alpha 2 beta 1 integrin, they started to move laterally on cell surface along actin filaments. During the lateral movement small clusters fused together. Finally alpha 2 beta 1 integrin was found inside caveolae and subsequently internalized into caveosome-like perinuclear structures. The internalization process, unlike cluster formation or lateral redistribution, was dependent on protein kinase C alpha activity. Caveolae are known to be highly immobile structures and alpha 2 beta 1 integrin clusters represent a previously unknown mechanism to activate endocytic trafficking via caveolae. The process was specific to alpha 2 beta 1 integrin, because the antibody-mediated formation of alpha V integrin clusters activated their internalization in coated vesicles and early endosomes. In addition to natural ligands human echovirus-1 (EV1) gains entry into the cell by binding to alpha 2 beta 1 and taking advantage of alpha 2 beta 1 internalization via caveolae.
Figures


References
-
- Akula, S.M., Pramod, N.P., Wang, F.Z., and Chandran, B. (2002). Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108, 407-419. - PubMed
-
- Benmerah, A., Bayrou, M., Cerf-Bensussan, N., and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303-1311. - PubMed
-
- Bergelson, J.M., Shepley, M.P., Chan, B.M., Hemler, M.E., and Finberg, R.W. (1992). Identification of the integrin VLA-2 as a receptor for echovirus 1. Science 255, 1718-1720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

