The EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements
- PMID: 14657246
- PMCID: PMC329218
- DOI: 10.1091/mbc.e03-07-0500
The EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements
Abstract
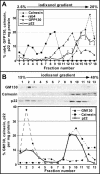
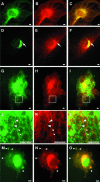
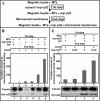
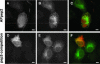
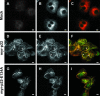
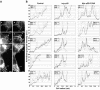
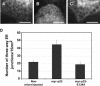
We have reported that p22, an N-myristoylated EF-hand Ca(2+)-binding protein, associates with microtubules and plays a role in membrane trafficking. Here, we show that p22 also associates with membranes of the early secretory pathway membranes, in particular endoplasmic reticulum (ER). On binding of Ca(2+), p22's ability to associate with membranes increases in an N-myristoylation-dependent manner, which is suggestive of a nonclassical Ca(2+)-myristoyl switch mechanism. To address the intracellular functions of p22, a digitonin-based "bulk microinjection" assay was developed to load cells with anti-p22, wild-type, or mutant p22 proteins. Antibodies against a p22 peptide induce microtubule depolymerization and ER fragmentation; this antibody-mediated effect is overcome by preincubation with the respective p22 peptide. In contrast, N-myristoylated p22 induces the formation of microtubule bundles, the accumulation of ER structures along the bundles as well as an increase in ER network formation. An N-myristoylated Ca(2+)-binding p22 mutant, which is unable to undergo Ca(2+)-mediated conformational changes, induces microtubule bundling and accumulation of ER structures along the bundles but does not increase ER network formation. Together, these data strongly suggest that p22 modulates the organization and dynamics of microtubule cytoskeleton in a Ca(2+)-independent manner and affects ER network assembly in a Ca(2+)-dependent manner.
Figures


References
-
- Allan, V.J., and Schroer, T.A. (1999). Membrane motors. Curr. Opin. Cell Biol. 11, 476-482. - PubMed
-
- Ames, J.B., Ishima, R., Tanaka, T., Gordon, J.I., Stryer, L., and Ikura, M. (1997). Molecular mechanics of calcium-myristoyl switches. Nature 389, 198-202. - PubMed
-
- Apodaca, G. (2001). Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2, 149-159. - PubMed
-
- Ashby, M.C., and Tepikin, A.V. (2002). Polarized calcium and calmodulin signaling in secretory epithelia. Physiol. Rev. 82, 701-34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

