In vitro analog of classical conditioning of feeding behavior in aplysia
- PMID: 14657259
- PMCID: PMC305463
- DOI: 10.1101/lm.65303
In vitro analog of classical conditioning of feeding behavior in aplysia
Abstract
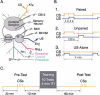
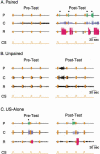
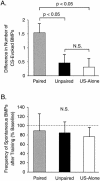
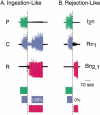
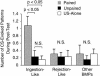
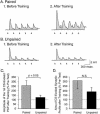
The feeding behavior of Aplysia californica can be classically conditioned using tactile stimulation of the lips as a conditioned stimulus (CS) and food as an unconditioned stimulus (US). Moreover, several neural correlates of classical conditioning have been identified. The present study extended previous work by developing an in vitro analog of classical conditioning and by investigating pairing-specific changes in neuronal and synaptic properties. The preparation consisted of the isolated cerebral and buccal ganglia. Electrical stimulation of a lip nerve (AT4) and a branch of the esophageal nerve (En2) served as the CS and US, respectively. Three protocols were used: paired, unpaired, and US alone. Only the paired protocol produced a significant increase in CS-evoked fictive feeding. At the cellular level, classical conditioning enhanced the magnitude of the CS-evoked synaptic input to pattern-initiating neuron B31/32. In addition, paired training enhanced both the magnitude of the CS-evoked synaptic input and the CS-evoked spike activity in command-like neuron CBI-2. The in vitro analog of classical conditioning reproduced all of the cellular changes that previously were identified following behavioral conditioning and has led to the identification of several new learning-related neural changes. In addition, the pairing-specific enhancement of the CS response in CBI-2 indicates that some aspects of associative plasticity may occur at the level of the cerebral sensory neurons.
Figures





Similar articles
-
Classical conditioning of feeding in Aplysia: II. Neurophysiological correlates.J Neurosci. 2000 May 1;20(9):3377-86. doi: 10.1523/JNEUROSCI.20-09-03377.2000. J Neurosci. 2000. PMID: 10777800 Free PMC article.
-
The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia.J Neurosci. 2001 Aug 15;21(16):6413-22. doi: 10.1523/JNEUROSCI.21-16-06413.2001. J Neurosci. 2001. PMID: 11487665 Free PMC article.
-
Classical conditioning of feeding in Aplysia: I. Behavioral analysis.J Neurosci. 2000 May 1;20(9):3369-76. doi: 10.1523/JNEUROSCI.20-09-03369.2000. J Neurosci. 2000. PMID: 10777799 Free PMC article.
-
Feeding behavior of Aplysia: a model system for comparing cellular mechanisms of classical and operant conditioning.Learn Mem. 2006 Nov-Dec;13(6):669-80. doi: 10.1101/lm.339206. Learn Mem. 2006. PMID: 17142299 Review.
-
The cellular basis of classical conditioning in Aplysia californica--it's less simple than you think.Trends Neurosci. 1995 Jan;18(1):30-6. doi: 10.1016/0166-2236(95)93947-v. Trends Neurosci. 1995. PMID: 7535488 Review.
Cited by
-
Extending in vitro conditioning in Aplysia to analyze operant and classical processes in the same preparation.Learn Mem. 2004 Jul-Aug;11(4):412-20. doi: 10.1101/lm.74404. Epub 2004 Jul 14. Learn Mem. 2004. PMID: 15254218 Free PMC article.
-
cGMP mediates short- and long-term modulation of excitability in a decision-making neuron in Aplysia.Neurosci Lett. 2018 Sep 14;683:111-118. doi: 10.1016/j.neulet.2018.06.046. Epub 2018 Jun 28. Neurosci Lett. 2018. PMID: 29960055 Free PMC article.
-
Localization of biogenic amines in the foregut of Aplysia californica: catecholaminergic and serotonergic innervation.J Comp Neurol. 2009 Jun 1;514(4):329-42. doi: 10.1002/cne.21991. J Comp Neurol. 2009. PMID: 19330814 Free PMC article.
-
Octopamine promotes rhythmicity but not synchrony in a bilateral pair of bursting motor neurons in the feeding circuit of Aplysia.J Exp Biol. 2010 Apr;213(Pt 7):1182-94. doi: 10.1242/jeb.040378. J Exp Biol. 2010. PMID: 20228355 Free PMC article.
-
Dopamine as a Multifunctional Neurotransmitter in Gastropod Molluscs: An Evolutionary Hypothesis.Biol Bull. 2020 Dec;239(3):189-208. doi: 10.1086/711293. Epub 2020 Nov 20. Biol Bull. 2020. PMID: 33347799 Free PMC article. Review.
References
-
- Abrams, T., Yovell, Y., Onyike, C., Cohen, J., and Jarrard, H. 1998. Analysis of sequence-dependent interaction between transient calcium and transmitter stimuli in activating adenylyl cyclase in Aplysia: Possible contribution to CS-US sequence requirement during conditioning. Learn. Mem. 4: 496-509. - PubMed
-
- Anderson, J.A. 1967. Patterns of response of neurons in the cerebral ganglion of Aplysia californica. Exp. Neurol. 19: 65-77. - PubMed
-
- Antonov, I., Antonova, I., Kandel, E.R., and Hawkins R.D. 2003. Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron 37: 135-147. - PubMed
-
- Balaban, P.M., Bravarenko, N.I., Maksimova, O.A., Nikitin, E., Ierusalimsky, V.N., and Zakharov, I.S. 2001. A single serotonergic modulatory cell can mediate reinforcement in the withdrawal network of the terrestrial snail. Neurobiol. Learn. Mem. 75: 30-50. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
