Measurements of the concentration and composition of nuclei for cirrus formation
- PMID: 14657330
- PMCID: PMC299754
- DOI: 10.1073/pnas.2532677100
Measurements of the concentration and composition of nuclei for cirrus formation
Abstract
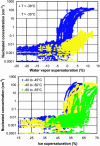
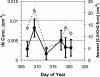
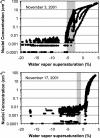
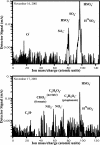
This article addresses the need for new data on indirect effects of natural and anthropogenic aerosol particles on atmospheric ice clouds. Simultaneous measurements of the concentration and composition of tropospheric aerosol particles capable of initiating ice in cold (cirrus) clouds are reported. Measurements support that cirrus formation occurs both by heterogeneous nucleation by insoluble particles and homogeneous (spontaneous) freezing of particles containing solutions. Heterogeneous ice nuclei concentrations in the cirrus regime depend on temperature, relative humidity, and the concentrations and physical and chemical properties of aerosol particles. The cirrus-active concentrations of heterogeneous nuclei measured in November over the western U.S. were <0.03 cm-3. Considering previous modeling studies, this result suggests a predominant potential impact of these nuclei on cirrus formed by slow, large-scale lifting or small cooling rates, including subvisual cirrus. The most common heterogeneous ice nuclei were identified as relatively pure mineral dusts and metallic particles, some of which may have origin through anthropogenic processes. Homogeneous freezing of large numbers of particles was detected above a critical relative humidity along with a simultaneous transition in nuclei composition toward that of the sulfate-dominated total aerosol population. The temperature and humidity conditions of the homogeneous nucleation transition were reasonably consistent with expectations based on previous theoretical and laboratory studies but were highly variable. The strong presence of certain organic pollutants was particularly noted to be associated with impedance of homogeneous freezing.
Figures


References
-
- Intergovernmental Panel on Climate Change (2001) in Climate Change 2001: The Scientific Basis, eds., Houghton, J. T., Ding, Y., Griggs, D. J., Noguer, M., van der Linden, P. J., Dai, X., Maskell, K. & Johnson, C. A. (Cambridge Univ. Press, Cambridge, U.K.), pp. 291–335.
-
- Liou, K.-N. (1986) Mon. Weather Rev. 114, 1167–1199.
-
- Stephens, G. L., Tsay, S., Stackhouse, P. W., Jr., & Flatau, P. J. (1990) J. Atmos. Sci. 47, 1742–1753.
-
- Lin, R.-F., Starr, D. O. C., DeMott, P. J., Cotton, R., Sassen, K., Jensen, E., Kärcher, B. & Liu, X. (2002) J. Atmos. Sci. 59, 2305–2329.
-
- DeMott, P. J., Rogers, D. C. & Kreidenweis, S. M. (1997) J. Geophys. Res. 102, 19575–19584.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

