The atherogenic effect of excess methionine intake
- PMID: 14657334
- PMCID: PMC299913
- DOI: 10.1073/pnas.2436385100
The atherogenic effect of excess methionine intake
Abstract
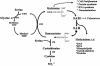
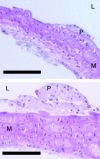
Methionine is the precursor of homocysteine, a sulfur amino acid intermediate in the methylation and transsulfuration pathways. Elevated plasma homocysteine (hyperhomocysteinemia) is associated with occlusive vascular disease. Whether homocysteine per se or a coincident metabolic abnormality causes vascular disease is still an open question. Animals with genetic hyperhomocysteinemia have so far not displayed atheromatous lesions. However, when methionine-rich diets are used to induce hyperhomocysteinemia, vascular pathology is often observed. Such studies have not distinguished the effects of excess dietary methionine from those of hyperhomocysteinemia. We fed apolipoprotein E-deficient mice with experimental diets designed to achieve three conditions: (i) high methionine intake with normal blood homocysteine; (ii) high methionine intake with B vitamin deficiency and hyperhomocysteinemia; and (iii) normal methionine intake with B vitamin deficiency and hyperhomocysteinemia. Mice fed methionine-rich diets had significant atheromatous pathology in the aortic arch even with normal plasma homocysteine levels, whereas mice fed B vitamin-deficient diets developed severe hyperhomocysteinemia without any increase in vascular pathology. Our findings suggest that moderate increases in methionine intake are atherogenic in susceptible mice. Although homocysteine may contribute to the effect of methionine, high plasma homocysteine was not independently atherogenic in this model. Some product of excess methionine metabolism rather than high plasma homocysteine per se may underlie the association of homocysteine with vascular disease.
Figures

Similar articles
-
Sulfur amino acids and atherosclerosis: a role for excess dietary methionine.Ann N Y Acad Sci. 2016 Jan;1363:18-25. doi: 10.1111/nyas.12962. Epub 2015 Dec 8. Ann N Y Acad Sci. 2016. PMID: 26647293
-
Effects of vitamin supplementation and hyperhomocysteinemia on atherosclerosis in apoE-deficient mice.Atherosclerosis. 2003 Jun;168(2):255-62. doi: 10.1016/s0021-9150(03)00138-2. Atherosclerosis. 2003. PMID: 12801608
-
Plasma S-adenosylhomocysteine is a better biomarker of atherosclerosis than homocysteine in apolipoprotein E-deficient mice fed high dietary methionine.J Nutr. 2008 Feb;138(2):311-5. doi: 10.1093/jn/138.2.311. J Nutr. 2008. PMID: 18203897
-
[Hyperhomocysteinemia: an independent risk factor or a simple marker of vascular disease?. 1. Basic data].Pathol Biol (Paris). 2003 Mar;51(2):101-10. doi: 10.1016/s0369-8114(03)00104-4. Pathol Biol (Paris). 2003. PMID: 12801808 Review. French.
-
DACH-LIGA homocystein (german, austrian and swiss homocysteine society): consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: guidelines and recommendations.Clin Chem Lab Med. 2003 Nov;41(11):1392-403. doi: 10.1515/CCLM.2003.214. Clin Chem Lab Med. 2003. PMID: 14656016 Review.
Cited by
-
Excess dietary methionine does not affect fracture healing in mice.Med Sci Monit. 2012 Dec;18(12):BR469-74. doi: 10.12659/msm.883590. Med Sci Monit. 2012. PMID: 23197225 Free PMC article.
-
Tangshen formula improves diabetic nephropathy in STZ-induced diabetes rats fed with hyper-methionine by regulating the methylation status of kidney.Clin Epigenetics. 2024 Jan 2;16(1):1. doi: 10.1186/s13148-023-01620-8. Clin Epigenetics. 2024. PMID: 38167534 Free PMC article.
-
A Hypomethylating Ketogenic Diet in Apolipoprotein E-Deficient Mice: A Pilot Study on Vascular Effects and Specific Epigenetic Changes.Nutrients. 2021 Oct 13;13(10):3576. doi: 10.3390/nu13103576. Nutrients. 2021. PMID: 34684577 Free PMC article.
-
Effect of methionine dietary supplementation on mitochondrial oxygen radical generation and oxidative DNA damage in rat liver and heart.J Bioenerg Biomembr. 2009 Jun;41(3):309-21. doi: 10.1007/s10863-009-9229-3. Epub 2009 Jul 25. J Bioenerg Biomembr. 2009. PMID: 19633937
-
PAI-1 and homocysteine, but not lipoprotein (a) and thrombophilic polymorphisms, are independently associated with the occurrence of major adverse cardiac events after successful coronary stenting.Heart. 2006 Mar;92(3):377-81. doi: 10.1136/hrt.2005.061895. Epub 2005 Jul 1. Heart. 2006. PMID: 15994914 Free PMC article.
References
-
- Bautista, L. E., Arenas, I. A., Penuela, A. & Martinez, L. X. (2002) J. Clin. Epidemiol. 55, 882-887. - PubMed
-
- Boushey, C. J., Beresford, S. A., Omenn, G. S. & Motulsky, A. G. (1995) J. Am. Med. Assoc. 274, 1049-1057. - PubMed
-
- Homocysteine Studies Collaboration. (2002) J. Am. Med. Assoc. 288, 2015-2022. - PubMed
-
- Eikelboom, J. W., Lonn, E., Genest, J., Jr., Hankey, G. & Yusuf, S. (1999) Ann. Intern. Med. 131, 363-375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

