Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces
- PMID: 14657343
- PMCID: PMC299759
- DOI: 10.1073/pnas.2434367100
Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces
Abstract
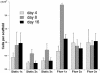
In this study we report on direct involvement of fluid shear stresses on the osteoblastic differentiation of marrow stromal cells. Rat bone marrow stromal cells were seeded in 3D porous titanium fiber mesh scaffolds and cultured for 16 days in a flow perfusion bioreactor with perfusing culture media of different viscosities while maintaining the fluid flow rate constant. This methodology allowed exposure of the cultured cells to increasing levels of mechanical stimulation, in the form of fluid shear stress, whereas chemotransport conditions for nutrient delivery and waste removal remained essentially constant. Under similar chemotransport for the cultured cells in the 3D porous scaffolds, increasing fluid shear forces led to increased mineral deposition, suggesting that the mechanical stimulation provided by fluid shear forces in 3D flow perfusion culture can indeed enhance the expression of the osteoblastic phenotype. Increased fluid shear forces also resulted in the generation of a better spatially distributed extracellular matrix inside the porosity of the 3D titanium fiber mesh scaffolds. The combined effect of fluid shear forces on the mineralized extracellular matrix production and distribution emphasizes the importance of mechanosensation on osteoblastic cell function in a 3D environment.
Figures




References
-
- Buckwalter, J. A., Glimcher, M. J., Cooper, R. R. & Recker, R. (1995) J. Bone Joint Surg. 77, 1256–1289.
-
- Klein-Nulend, J., van der Plas, A., Semeins, C. M., Ajubi, N. E., Frangos, J. A., Nijweide, P. J. & Burger, E. H. (1995) FASEB J. 9, 441–445. - PubMed
-
- Owan, I., Burr, D. B., Turner, C. H., Qiu, J., Tu, Y., Onyia, J. E. & Duncan, R. L. (1997) Am. J. Physiol. 273, C810–C815. - PubMed
-
- Smalt, R., Mitchell, F. T., Howard, R. L. & Chambers, T. J. (1997) Am. J. Physiol. 273, E751–E758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

