The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart
- PMID: 14657344
- PMCID: PMC299971
- DOI: 10.1073/pnas.2434235100
The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart
Abstract
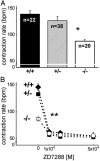
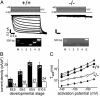
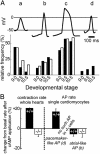
Hyperpolarization-activated, cyclic nucleotide-gated cation currents, termed If or Ih, are generated by four members of the hyperpolarization-activated, cyclic nucleotide-gated cation (HCN) channel family. These currents have been proposed to contribute to several functions including pacemaker activity in heart and brain, control of resting potential, and neuronal plasticity. Transcripts of the HCN4 isoform have been found in cardiomyocytes and neurons, but the physiological role of this channel is unknown. Here we show that HCN4 is essential for the proper function of the developing cardiac conduction system. In wild-type embryos, HCN4 is highly expressed in the cardiac region where the early sinoatrial node develops. Mice lacking HCN4 channels globally, as well as mice with a selective deletion of HCN4 in cardiomyocytes, died between embryonic days 9.5 and 11.5. On average, If in cardiomyocytes from mutant embryos is reduced by 85%. Hearts from HCN4-deficient embryos contracted significantly slower compared with wild type and could not be stimulated by cAMP. In both wild-type and HCN4-/- mice, cardiac cells with "primitive" pacemaker action potentials could be found. However, cardiac cells with "mature" pacemaker potentials, observed in wild-type embryos starting at day 9.0, were not detected in HCN4-deficient embryos. Thus, HCN4 channels are essential for the proper generation of pacemaker potentials in the emerging sinoatrial node.
Figures


References
-
- Biel, M., Ludwig, A., Zong, X. & Hofmann, F. (1999) Rev. Physiol. Biochem. Pharmacol. 136, 165-181. - PubMed
-
- Santoro, B. & Tibbs, G. R. (1999) Ann. N.Y. Acad. Sci. 868, 741-764. - PubMed
-
- Kaupp, U. B. & Seifert, R. (2001) Annu. Rev. Physiol. 63, 235-257. - PubMed
-
- DiFrancesco, D. (1993) Annu. Rev. Physiol. 55, 455-472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

