A class of sterol 14-demethylase inhibitors as anti-Trypanosoma cruzi agents
- PMID: 14657358
- PMCID: PMC299928
- DOI: 10.1073/pnas.2535442100
A class of sterol 14-demethylase inhibitors as anti-Trypanosoma cruzi agents
Abstract
Chronic infection with the protozoan parasite Trypanosoma cruzi is a major cause of morbidity and mortality in Latin America. Drug treatments for the associated illness, Chagas disease, are toxic and frequently unsuccessful. In a screening effort against the drug target protein farnesyltransferase, we identified a series of disubstituted imidazoles with highly potent anti-T. cruzi activity that apparently acted through a mechanism independent of protein farnesylation. Metabolic labeling studies of T. cruzi suggested that sterol biosynthesis was inhibited. Combined GC/MS analysis confirmed depletion of cellular sterols and suggested that the site of action was sterol 14-demethylase, a cytochrome P450 enzyme. Spectral studies with recombinant T. cruzi sterol 14-demethylase demonstrated that the compounds bind directly to this enzyme. Two of the compounds were well absorbed when given orally to mice, gave sustained plasma levels, and were well tolerated. The compounds were administered orally to mice with acute T. cruzi infection and caused dramatic decrease in parasitemia and led to 100% survival. These disubstituted imidazole compounds can be prepared by a relatively short synthetic route and represent a structural class with potent anti-T. cruzi activity.
Figures
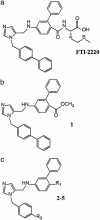

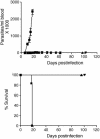
References
-
- World Health Organization (2002) Control of Chagas Disease: Second Report of the Expert Committee (W.H.O., Geneva), W.H.O. Technical Report Series 905.
-
- Buckner, F. S., Eastman, R., Speelmon, E., Nepomuceno-Silva, J. L., Myler P., Van Voorhis, W. C. & Yokoyama, K. (2002) Mol. Biochem. Parasitol. 122, 181-188. - PubMed
-
- Haluska, P., Dy, G. K. & Adjei, A. A. (2002) Eur. J. Cancer 38, 1685-1700. - PubMed
-
- Gelb, M. H., Buckner, F. S., Yokoyama, K., Ohkanda, J., Hamilton, A., Hguyen, L., Rossi-Bergmann, B., Sebti, S. M. & Van Voorhis, W. C. (2000) in Farnesyltransferase and Geranylgeranyltransferase-I: Targets for Cancer and Cardiovascular Therapy, ed. Sebti, S. M. (Humana, Totowa, NJ), pp. 221-232.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

