Hyperactivated sperm motility driven by CatSper2 is required for fertilization
- PMID: 14657366
- PMCID: PMC299835
- DOI: 10.1073/pnas.2136654100
Hyperactivated sperm motility driven by CatSper2 is required for fertilization
Abstract
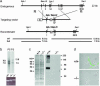
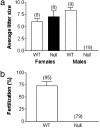
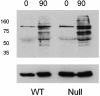
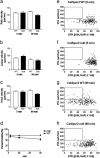
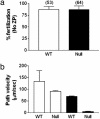
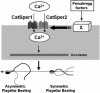
Elevations of sperm Ca2+ seem to be responsible for an asymmetric form of motility called hyperactivation, which is first seen near the time of fertilization. The mechanism by which intracellular Ca2+ concentrations increase remains unknown despite considerable investigation. Although several prototypical voltage-gated calcium channels are present in spermatozoa, they are not essential for motility. Furthermore, the forward velocity and percentage of motility of spermatozoa are associated with infertility, but their importance relative to hyperactivation also remains unknown. We show here that disruption of the gene for a recently described sperm-specific voltage-gated cation channel, CatSper2, fails to significantly alter sperm production, protein tyrosine phosphorylation that is associated with capacitation, induction of the acrosome reaction, forward velocity, or percentage of motility, yet CatSper2-/- males are completely infertile. The defect that we identify in the null sperm cells is a failure to acquire hyperactivated motility, which seems to render spermatozoa incapable of generating the "power" needed for penetration of the extracellular matrix of the egg. A loss of power is suggested also by experiments in which the viscosity of the medium was increased after incubation of spermatozoa in normal capacitating conditions. In high-viscosity medium, CatSper2-null spermatozoa lost the ability to swim forward, whereas wild-type cells continued to move forward. Thus, CatSper2 is responsible for driving hyperactivated motility, and, even with typical sperm forward velocities, fertilization is not possible in the absence of this highly active form of motility.
Figures
References
-
- Wennemuth, G., Westenbroek, R. E., Xu, T., Hille, B. & Babcock, D. F. (2000) J. Biol. Chem. 275, 21210–21217. - PubMed
-
- Castellano, L. E., Trevino, C. L., Rodriguez, D., Serrano, C. J., Pacheco, J., Tsutsumi, V., Felix, R. & Darszon, A. (2003) FEBS Lett. 541, 69–74. - PubMed
-
- Jungnickel, M. K., Marrero, H., Birnbaumer, L., Lemos, J. R. & Florman, H. M. (2001) Nat. Cell Biol. 3, 499–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

