Thermodynamic stability of hydrogen clathrates
- PMID: 14657391
- PMCID: PMC299752
- DOI: 10.1073/pnas.2430913100
Thermodynamic stability of hydrogen clathrates
Abstract
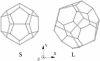
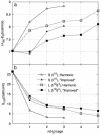
The stability of the recently characterized type II hydrogen clathrate [Mao, W. L., Mao, H.-K., Goncharov, A. F., Struzhkin, V. V., Guo, Q., et al. (2002) Science 297, 2247-2249] with respect to hydrogen occupancy is examined with a statistical mechanical model in conjunction with first-principles quantum chemistry calculations. It is found that the stability of the clathrate is mainly caused by dispersive interactions between H2 molecules and the water forming the cage walls. Theoretical analysis shows that both individual hydrogen molecules and nH2 guest clusters undergo essentially free rotations inside the clathrate cages. Calculations at the experimental conditions--2,000 bar (1 bar = 100 kPa) and 250 K confirm multiple occupancy of the clathrate cages with average occupations of 2.00 and 3.96 H2 molecules per D-5(12) (small) and H-5(12)6(4) (large) cage, respectively. The H2-H2O interactions also are responsible for the experimentally observed softening of the H[bond]H stretching modes. The clathrate is found to be thermodynamically stable at 25 bar and 150 K.
Figures

References
-
- Jeffrey, G. A. (1984) in Inclusion Compounds, eds. Atwood, J. L., Davies, J. E. D. & MacNicol, D. D. (Academic, New York).
-
- Suess, E., Bohrmann, G., Greinert, J. & Lausch, E. (1999) Sci. Am. 281 (5), 52–59.
-
- Sloan, E. D. (2000) Hydrate Engineering Monograph (Soc. Plastic Engineer., Richardson, TX), Vol. 21.
-
- Katz, M. E., Pak, D. K., Dickens, G. R. & Miller, K. G. (1999) Science 286, 1531–1533. - PubMed
-
- Hessello, S. P. P., Grocke, D. R., Jenkyns, K. C., Bjerrum, C. J., Farrimond, P., Bell, H. S. M. & Green, O. R. (2000) Nature 406, 392–395. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

