A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1)
- PMID: 14657394
- PMCID: PMC299797
- DOI: 10.1073/pnas.2336107100
A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1)
Abstract
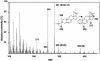
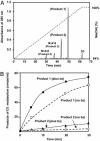
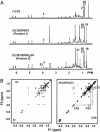
Metabolites of vitamin D3 (D3) (cholecalciferol) are recognized as enzymatically formed chemicals in humans that can influence a wide variety of reactions that regulate a large number of cellular functions. The metabolism of D3 has been extensively studied, and a role for three different mitochondrial cytochrome P450s (CYP24A, CYP27A, and CYP27B1) has been described that catalyze the formation of the 24(OH), 25(OH), and 1(OH) metabolites of D3, respectively. The hormone 1,25-dihydroxyvitamin D3 has been most extensively studied and is widely recognized as a regulator of calcium and phosphorous metabolism. Hydroxylated metabolites of D3 interact with the nuclear receptor and thereby influence growth, cellular differentiation, and proliferation. In this article, we describe in vitro experiments using purified mitochondrial cytochrome P450scc (CYP11A1) reconstituted with the iron-sulfer protein, adrenodoxin, and the flavoprotein, adrenodoxin reductase, and show the NADPH and time-dependent formation of two major metabolites of D3 (i.e., 20-hydroxyvitamin D3 and 20,22-dihydroxyvitamin D3) plus two unknown minor metabolites. In addition, we describe the metabolism of 7-dehydrocholesterol by CYP11A1 to a single product identified as 7-dehydropregnenolone. Although the physiological importance of these hydroxylated metabolites of D3 and their in vivo formation and mode of action remain to be determined, the rate with which they are formed by CYP11A1 in vitro suggests an important role.
Figures


References
-
- Solomon, S., Levitan, P. & Lieberman, S. (1956) Rev. Can. Biol. 15, 282–283.
-
- Shimizu, K., Gut, M. & Dorfman, R. I. (1962) J. Biol. Chem. 237, 699–702. - PubMed
-
- Constantopoulos, G. & Tchen, T. T. (1961) Biochem. Biophys. Res. Commun. 4, 460–463. - PubMed
-
- Hume, R., Kelly, R. W., Taylor, P. L. & Boyd, G. S. (1984) Eur. J. Biochem. 140, 583–591. - PubMed
-
- Burstein, S. & Gut, M. (1976) Steroids 28, 115–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

