A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation
- PMID: 14657396
- PMCID: PMC299823
- DOI: 10.1073/pnas.2335201100
A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation
Abstract
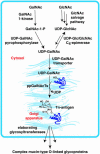
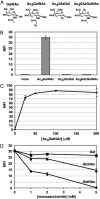
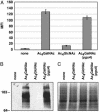
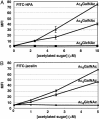
Mucin-type O-linked glycoproteins are involved in a variety of biological interactions in higher eukaryotes. The biosynthesis of these glycoproteins is initiated by a family of polypeptide N-acetyl-alpha-galactosaminyltransferases (ppGalNAcTs) that modify proteins in the secretory pathway. The lack of a defined consensus sequence for the ppGalNAcTs makes the prediction of mucin-type O-linked glycosylation difficult based on primary sequence alone. Herein we present a method for labeling mucin-type O-linked glycoproteins with a unique chemical tag, the azide, which permits their selective covalent modification from complex cell lysates. From a panel of synthetic derivatives, we identified an azido GalNAc analog (N-azidoacetylgalactosamine, GalNAz) that is metabolized by numerous cell types and installed on mucin-type O-linked glycoproteins by the ppGalNAcTs. The azide serves as a bioorthogonal chemical handle for selective modification with biochemical or biophysical probes using the Staudinger ligation. The approach was validated by labeling a recombinant glycoprotein that is known to possess O-linked glycans with GalNAz. In addition, GalNAz efficiently labeled mucin-type O-linked glycoproteins expressed at endogenous levels. The ability to label mucin-type O-linked glycoproteins with chemical tags should facilitate their identification by proteomic strategies.
Figures


References
-
- Mann, M. & Jensen, O. N. (2003) Nat. Biotechnol. 21, 255–261. - PubMed
-
- Bill, R. M., Revers, L. & Wilson, I. A. (1998) Protein Glycosylation (Kluwer Academic, Boston).
-
- Rudd, P. M. & Dwek, R. A. (1997) Crit. Rev. Biochem. Mol. Biol. 32, 1–100. - PubMed
-
- Lowe, J. B. & Marth, J. D. (2003) Annu. Rev. Biochem. 72, 643–691. - PubMed
-
- Hirabayashi, J. (2003) Trends Biotechnol. 21, 141–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

