Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e)
- PMID: 14657401
- PMCID: PMC316292
- DOI: 10.1104/pp.103.025379
Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e)
Abstract
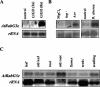
Adaptation to stress requires removal of existing molecules from various cellular compartments and replacing them with new ones. The transport of materials to and from the specific compartments involved in the recycling and deposition of macromolecules is carried out by an intracellular vesicle trafficking system. Here, we report the isolation of a vesicle trafficking-regulating gene, AtRabG3e (formerly AtRab7), from Arabidopsis. The gene was induced during programmed cell death after treatment of intact leaves with superoxide and salicylic acid or infection with necrogenic pathogens. Transgenic plants that expressed the AtRabG3e gene under the constitutive 35S promoter from cauliflower mosaic virus exhibited accelerated endocytosis in roots, leaves, and protoplasts. The transgenic plants accumulated sodium in the vacuoles and had higher amounts of sodium in the shoots. The transgenic plants also showed increased tolerance to salt and osmotic stresses and reduced accumulation of reactive oxygen species during salt stress. These results imply that vesicle trafficking plays an important role in plant adaptation to stress, beyond the housekeeping function in intracellular vesicle trafficking.
Figures
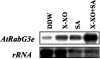







Similar articles
-
The RING finger E3 ligase STRF1 is involved in membrane trafficking and modulates salt-stress response in Arabidopsis thaliana.Plant J. 2015 Apr;82(1):81-92. doi: 10.1111/tpj.12797. Epub 2015 Mar 7. Plant J. 2015. PMID: 25704231
-
Cloning of a vacuolar H(+)-pyrophosphatase gene from the halophyte Suaeda corniculata whose heterologous overexpression improves salt, saline-alkali and drought tolerance in Arabidopsis.J Integr Plant Biol. 2011 Sep;53(9):731-42. doi: 10.1111/j.1744-7909.2011.01066.x. J Integr Plant Biol. 2011. PMID: 21762382
-
Isolation and functional characterization of the Arabidopsis salt-tolerance 32 (AtSAT32) gene associated with salt tolerance and ABA signaling.Physiol Plant. 2009 Apr;135(4):426-35. doi: 10.1111/j.1399-3054.2008.01202.x. Epub 2009 Feb 5. Physiol Plant. 2009. PMID: 19210750
-
Salt tolerance-related protein STO binds to a Myb transcription factor homologue and confers salt tolerance in Arabidopsis.J Exp Bot. 2003 Oct;54(391):2231-7. doi: 10.1093/jxb/erg241. Epub 2003 Aug 8. J Exp Bot. 2003. PMID: 12909688
-
Vesicle trafficking in rice: too little is known.Front Plant Sci. 2023 Sep 18;14:1263966. doi: 10.3389/fpls.2023.1263966. eCollection 2023. Front Plant Sci. 2023. PMID: 37790794 Free PMC article. Review.
Cited by
-
The Tip-Localized Phosphatidylserine Established by Arabidopsis ALA3 Is Crucial for Rab GTPase-Mediated Vesicle Trafficking and Pollen Tube Growth.Plant Cell. 2020 Oct;32(10):3170-3187. doi: 10.1105/tpc.19.00844. Epub 2020 Aug 18. Plant Cell. 2020. PMID: 32817253 Free PMC article.
-
Arabidopsis response to low-phosphate conditions includes active changes in actin filaments and PIN2 polarization and is dependent on strigolactone signalling.J Exp Bot. 2015 Mar;66(5):1499-510. doi: 10.1093/jxb/eru513. Epub 2015 Jan 21. J Exp Bot. 2015. PMID: 25609825 Free PMC article.
-
Role of Arabidopsis RAB5 GEF vps9a in maintaining potassium levels under sodium chloride stress.Plant Direct. 2020 Oct 21;4(10):e00273. doi: 10.1002/pld3.273. eCollection 2020 Oct. Plant Direct. 2020. PMID: 33103044 Free PMC article.
-
Subcellular localization and functional analysis of the Arabidopsis GTPase RabE.Plant Physiol. 2009 Apr;149(4):1824-37. doi: 10.1104/pp.108.132092. Epub 2009 Feb 20. Plant Physiol. 2009. PMID: 19233904 Free PMC article.
-
Comparative phylogenetic analysis of small GTP-binding genes of model legume plants and assessment of their roles in root nodules.J Exp Bot. 2008;59(14):3831-44. doi: 10.1093/jxb/ern223. Epub 2008 Oct 9. J Exp Bot. 2008. PMID: 18849296 Free PMC article.
References
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 258: 1256-1258 - PubMed
-
- Bartels D, Nelson D (1994) Approaches to improve stress tolerance using molecular studies. Plant Cell Environ 17: 659-667
-
- Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12: 431-434 - PubMed
-
- Bolte S, Schiene K, Dietz KJ (2000) Characterization of a small GTP-binding protein of the rab 5 family in Mesembryanthemum crystallinum with increased level of expression during early salt stress. Plant Mol Biol 42: 923-936 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

