Expressed sequence tag profiling identifies developmental and anatomic partitioning of gene expression in the mouse prostate
- PMID: 14659016
- PMCID: PMC329418
- DOI: 10.1186/gb-2003-4-12-r79
Expressed sequence tag profiling identifies developmental and anatomic partitioning of gene expression in the mouse prostate
Abstract
Background: The prostate gland is an organ with highly specialized functional attributes that serves to enhance the fertility of mammalian species. Much of the information pertaining to normal and pathological conditions affecting the prostate has been obtained through extensive developmental, biochemical and genetic analyses of rodent species. Although important insights can be obtained through detailed anatomical and histological assessments of mouse and rat models, further mechanistic explanations are greatly aided through studies of gene and protein expression.
Results: In this article we characterize the repertoire of genes expressed in the normal developing mouse prostate through the analysis of 50,562 expressed sequence tags derived from 14 mouse prostate cDNA libraries. Sequence assemblies and annotations identified 15,009 unique transcriptional units of which more than 600 represent high quality assemblies without corresponding annotations in public gene expression databases. Quantitative analyses demonstrate distinct anatomical and developmental partitioning of prostate gene expression. This finding may assist in the interpretation of comparative studies between human and mouse and guide the development of new transgenic murine disease models. The identification of several novel genes is reported, including a new member of the beta-defensin gene family with prostate-restricted expression.
Conclusions: These findings suggest a potential role for the prostate as a defensive barrier for entry of pathogens into the genitourinary tract and, further, serve to emphasize the utility of the continued evaluation of transcriptomes from a diverse repertoire of tissues and cell types.
Figures


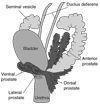



References
-
- Marengo SR. Prostate Physiology and Regulation. In: Resnick MI, Thompson IM, editor. Advanced Therapy of Prostate Disease. Hamilton/London: BC Decker, Inc; 2000. pp. 92–117.
-
- Yatani R, Kusano I, Shiraishi T, Hayashi T, Stemmermann GN. Latent prostatic carcinoma: Pathological and epidemiological aspects. Jpn J Clin Oncol. 1989;19:319–326. - PubMed
-
- Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47:273–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

