Variation in biofilm formation among strains of Listeria monocytogenes
- PMID: 14660383
- PMCID: PMC309931
- DOI: 10.1128/AEM.69.12.7336-7342.2003
Variation in biofilm formation among strains of Listeria monocytogenes
Abstract
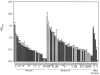
Contamination of food by Listeria monocytogenes is thought to occur most frequently in food-processing environments where cells persist due to their ability to attach to stainless steel and other surfaces. Once attached these cells may produce multicellular biofilms that are resistant to disinfection and from which cells can become detached and contaminate food products. Because there is a correlation between virulence and serotype (and thus phylogenetic division) of L. monocytogenes, it is important to determine if there is a link between biofilm formation and disease incidence for L. monocytogenes. Eighty L. monocytogenes isolates were screened for biofilm formation to determine if there is a robust relationship between biofilm formation, phylogenic division, and persistence in the environment. Statistically significant differences were detected between phylogenetic divisions. Increased biofilm formation was observed in Division II strains (serotypes 1/2a and 1/2c), which are not normally associated with food-borne outbreaks. Differences in biofilm formation were also detected between persistent and nonpersistent strains isolated from bulk milk samples, with persistent strains showing increased biofilm formation relative to nonpersistent strains. There were no significant differences detected among serotypes. Exopolysaccharide production correlated with cell adherence for high-biofilm-producing strains. Scanning electron microscopy showed that a high-biofilm-forming strain produced a dense, three-dimensional structure, whereas a low-biofilm-forming strain produced a thin, patchy biofilm. These data are consistent with data on persistent strains forming biofilms but do not support a consistent relationship between enhanced biofilm formation and disease incidence.
Figures


References
-
- Bibb, W. F., B. G. Gellin, R. Weaver, B. Schwartz, B. D. Plikaytis, M. W. Reeves, R. W. Pinner, and C. V. Broome. 1990. Analysis of clinical and food-borne isolates of Listeria monocytogenes in the United States by multilocus enzyme electrophoresis and application of the method to epidemiologic investigations. Appl. Environ. Microbiol. 56:2133-2141. - PMC - PubMed
-
- Borucki, M. K., M. J. Krug, W. T. Muraoka, and D. R. Call. 2003. Discrimination among Listeria monocytogenes isolates using a mixed genome DNA microarray. Vet. Microbiol. 92:351-362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

