Enumeration and characterization of iron(III)-reducing microbial communities from acidic subsurface sediments contaminated with uranium(VI)
- PMID: 14660400
- PMCID: PMC310038
- DOI: 10.1128/AEM.69.12.7467-7479.2003
Enumeration and characterization of iron(III)-reducing microbial communities from acidic subsurface sediments contaminated with uranium(VI)
Abstract
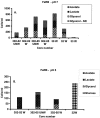
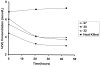
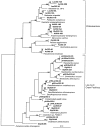
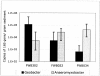
Iron(III)-reducing bacteria have been demonstrated to rapidly catalyze the reduction and immobilization of uranium(VI) from contaminated subsurface sediments. Thus, these organisms may aid in the development of bioremediation strategies for uranium contamination, which is prevalent in acidic subsurface sediments at U.S. government facilities. Iron(III)-reducing enrichment cultures were initiated from pristine and contaminated (high in uranium, nitrate; low pH) subsurface sediments at pH 7 and pH 4 to 5. Enumeration of Fe(III)-reducing bacteria yielded cell counts of up to 240 cells ml(-1) for the contaminated and background sediments at both pHs with a range of different carbon sources (glycerol, acetate, lactate, and glucose). In enrichments where nitrate contamination was removed from the sediment by washing, MPN counts of Fe(III)-reducing bacteria increased substantially. Sediments of lower pH typically yielded lower counts of Fe(III)-reducing bacteria in lactate- and acetate-amended enrichments, but higher counts were observed when glucose was used as an electron donor in acidic enrichments. Phylogenetic analysis of 16S rRNA gene sequences extracted from the highest positive MPN dilutions revealed that the predominant members of Fe(III)-reducing consortia from background sediments were closely related to members of the Geobacteraceae family, whereas a recently characterized Fe(III) reducer (Anaeromyxobacter sp.) and organisms not previously shown to reduce Fe(III) (Paenibacillus and Brevibacillus spp.) predominated in the Fe(III)-reducing consortia of contaminated sediments. Analysis of enrichment cultures by terminal restriction fragment length polymorphism (T-RFLP) strongly supported the cloning and sequencing results. Dominant members of the Fe(III)-reducing consortia were observed to be stable over several enrichment culture transfers by T-RFLP in conjunction with measurements of Fe(III) reduction activity and carbon substrate utilization. Enrichment cultures from contaminated sites were also shown to rapidly reduce millimolar amounts of U(VI) in comparison to killed controls. With DNA extracted directly from subsurface sediments, quantitative analysis of 16S rRNA gene sequences with MPN-PCR indicated that Geobacteraceae sequences were more abundant in pristine compared to contaminated environments,whereas Anaeromyxobacter sequences were more abundant in contaminated sediments. Thus, results from a combination of cultivation-based and cultivation-independent approaches indicate that the abundance/community composition of Fe(III)-reducing consortia in subsurface sediments is dependent upon geochemical parameters (pH, nitrate concentration) and that microorganisms capable of producing spores (gram positive) or spore-like bodies (Anaeromyxobacter) were representative of acidic subsurface environments.
Figures



References
-
- Achtnich, C., A. Schuhmann, T. Wind, and R. Conrad. 1995. Role of interspecies H2 transfer to sulfate and ferric iron-reducing bacteria in acetate consumption in anoxic paddy soil. FEMS Microbiol. Ecol. 16:61-70.
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- American Public Health Association. 1969. Standard methods for the examination of water and wastewater, including bottom sediments and sludge. American Public Health Association, Washington, D.C.
-
- Braman, R. S., and S. A. Hendrix. 1989. Nanogram nitrate and nitrite determination in environmental and biological materials by vanadium(III) reduction with chemiluminescence detection. Anal. Chem. 61:2715-2718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

