Is manual counting of corneal endothelial cell density in eye banks still acceptable? The French experience
- PMID: 14660458
- PMCID: PMC1920580
- DOI: 10.1136/bjo.87.12.1481
Is manual counting of corneal endothelial cell density in eye banks still acceptable? The French experience
Abstract
Aim: To examine the differences in manual endothelial cell counting methods in French eye banks and to analyse whether these differences could explain some substantial discrepancies observed in endothelial cell density (ECD) for corneas made available for transplant.
Methods: A questionnaire was sent to the 22 eye banks asking for details of the technical features of the light microscopes used, the microscope calibration, strategy for cell counting, the technical staff, and the method of presenting endothelial data.
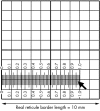
Results: All eye banks responded and 91% (20/22) used only manual counting methods, in real time, directly through a microscope, and 62 different technicians, with varying experience, were involved in such counting. Counting of cells within the borders of a grid that were in contact with two adjacent borders was the most common method (17/22, 77%). Of the eight banks (8/22, 36%) that did not calibrate their microscopes, six reported the highest ECD values. Of the 14 others (64%), six applied a "magnification correcting factor" to the initial cell counts. In five of these cases, the corrected ECD was lower than estimated on initial count. Most of the banks (12/22, 55%) counted 100 cells or less in one to six non-adjacent zones of the mosaic. 14 of the banks (14/22, 64%) also graded cell polymegethism while seven (7/22, 32%) also graded pleomorphism ("hexagonality").
Conclusions: Lack of microscope calibration appears to be the leading cause of variance in ECD estimates in French eye banks. Other factors such as differences in counting strategy, the evaluation of smaller numbers of cells, and the different extent of experience of the technicians may also contribute to intraobserver and interobserver variability. Further comparative studies, including cross checking and the outcome of repeated counts from manual methods, are clearly needed with cross calibration to a computer based image archiving and analysis system.
Figures


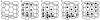
References
-
- EEBA. European Eye Bank Association Directory. 10th ed, 2002.
-
- Pels E, Schuchard Y. Organ-culture preservation of human corneas. Doc Ophthalmol 1983;56:147–53. - PubMed
-
- De Boroviczeny CG. On the standardization of the blood cell counts. Bibl Haematol 1966;24:2–31. - PubMed
-
- French directory of corneal storage centres.7th ed. Besançon, 1999.
-
- French directory of corneal storage centres.8th ed. Besançon, 2000.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
